Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 n.12 Johannesburg Jan. 2013
GENERAL PAPERS
Influence of particle shape on the flotation of magnetite, alone and in the presence of quartz particles
F. Dehghani; M. Rahimi; B. Rezai
Department of Mining and Metallurgical Engineering, Amirkabir University of Technology, Tehran, Iran
SYNOPSIS
The influence of different grinding methods on the shape characteristics of magnetite particles was investigated. Also, the effect of particle shape on the flotation of the magnetite, both alone and in the presence of quartz particles, was investigated. The shape characteristics of the magnetite particles were measured and calculated from images obtained by scanning electron microscopy via an image analysis system. The flotation tests were performed using a laboratory flotation cell. Results showed that in the -250 +212 µm and -106+75 µm size fractions, ball mill products have a higher elongation value than rod mill products, while in the -150+125 µm size fraction, rod mill products have a higher elongation value. Particles with higher elongation values and lower roundness values have a higher flotation kinetic constant. Furthermore, the influence of magnetite particle shape on flotation is greater, and the flotation kinetic constant is higher, in the presence of quartz particles than in the flotation of magnetite alone
Keywords: shape, elongation value, flotation kinetics, magnetite.
Introduction
In the flotation process, cumulative recovery as a function of time can be considered as the flotation rate. Many empirical models have been proposed by different researchers for flotation kinetics (e.g., Kuopanportti et al., 2000; Klimpel et al., 2000). Flotation kinetics can be expressed as a first-order rate equation (Xu, 1998; Oliveira et al., 2001; Agar et al., 1998; Cilek, 2004):

where t is cumulative flotation time, R is cumulative recovery after time t, R* is maximum theoretical flotation recovery, and k is the rate or kinetic constant (1/time).
The plot of  vs. time is a straight line, and the slope of this line is the first-order rate constant.
vs. time is a straight line, and the slope of this line is the first-order rate constant.
The particle shape characteristics will affect the physical, chemical, and surface properties of particles and therefore affect downstream mineral processing operations such as flotation.Mineral separation methods are very sensitive to particle shape parameters (Higyilmaz et al., 2004; Durney et al., 1986).
The distribution of particle shape has great significance in many industries such as civil engineering, chemical engineering, pharmaceuticals, and mining (Ahmed, 2010). Although the morphological properties of particles such as surface roughness and particle shape characteristics have a significant influence on flotation behaviour, few researchers have studied these effects (Kursun et al., 2006).
The initial studies on particle shape parameters were performed by Huh et al. (1974) and Forssberg et al. (1985). Huh et al. concluded that the adhesion force between particles and bubbles depends on particle shape parameters.
While conducting microflotation tests on quartz, calcite, and barite particles, Higyilmaz et al. (2004) and Ulusoy et al. (2004) observed that decreasing the elongation of particles and increasing the roundness increased the wettability . Kursun et al. (2006) showed that greater elongation and flatness had a positive effect on column flotation recovery of talc particles, while increased roundness and relative width had a negative effect.
Rahimi et al. (2012a,b) studied the effect of morphological parameters such as surface roughness and particle shape parameters on the flotation kinetics of quartz. They found that the particles with a higher surface roughness and higher elongation ratio had faster flotation kinetics. Furthermore, the influence of surface roughness on flotation kinetics was found to be greater than that of particle shape.
In this paper, the influence of particle shape on the flotation kinetics of magnetite, alone and in the presence of quartz particles, is investigated. Also, the time parameter is considered during recovery. Furthermore, particle-particle interaction, which has not been studied by previous researchers, is investigated.
Materials and method
Materials
A representative sample of magnetite was obtained from Yazd Province, Iran, and a representative sample of quartz was obtained from Ghazvin Province, Iran. Chemical and mineralogical analyses were carried out by X-ray fluorescence (XRF) spectrometry and X-ray diffraction (XRD). The magnetite sample contained 71% Fe3O4, and the quartz sample contained 99.4% SiO2. These samples were therefore pure enough for this study.
Tetrasodium N-( 1,2-dicarboxyethyl -N- octadecylsulfos-uccinamate), an anionic collector with the trade name Aero 845, was used for the flotation tests.
Grinding tests
The magnetite and quartz samples were crushed in a jaw crusher, a cone crusher, and a roll crusher to a product size of -2380+841 µm. The samples were then ground in laboratory-scale Denver ball and rod mills. The ball mill was 184 mm in length and 200 mm in internal diameter. The ball milling time was 45 minutes, and the sample weight was 1000 g. Rod milling was carried out using a laboratory mill 360 mm in length and 150 mm in internal diameter. The rod milling time was 55 minutes, and the sample weight was 1000 g. After grinding, the samples were wet sieved in a vibrating sieve shaker for 15 minutes for screen analysis, and the -250+212, -150+125, and -106+75 µm size fractions were selected for the next stages of the investigation.
Particle shape measurement
The shape parameters of the magnetite particles were measured with an XL30-Philips SEM. Representative samples from the -250+212, -150+125, and -106+75 µm size fractions of rod and ball mill products were used for SEM studies. The particles were coated with gold to provide conductivity. Images from different localities of the sample of each size fraction were taken at the appropriate magnifications. Then the length (L), width (W), area (A), and perimeter (P) of more than 100 particles in each representative sample were measured using a Clemex Vision PE image analysis system. The particles at the corners of the frame and overlapping particles were disregarded. Thus, by using the measured length, width, area, and perimeter, the elongation ratio
(ER) and roundness (Ro) were obtained from Equations [4] and [5] (Ulusoy et al., 2004; Rahimi et al., 2012a).




Figure 1 shows SEM images that were used for calculation of the elongation ratio (ER) and roundness (Ro) parameters.

Flotation tests
Flotation tests were performed in a laboratory Denver machine (model D12), using a 1.5 L cell. In these tests, from the three size fractions from the ball and rod mill products, magnetite particles were floated alone and in the presence of quartz particles.
Flotation of magnetite particles alone
The test conditions were 1000 r/min for rotor speed, 4 minutes for collector conditioning, and 70 seconds for froth removal until the froth was barren of magnetite. The weight of magnetite for each test was 100 g, and the water volume was 1.2 L.
A collector dosage of 750 g per ton of ore was used for each flotation test. The pH value for the magnetite flotation tests was 3.5.
Flotation of magnetite particles in the presence of quartz particles
The sample weight for each test was 50 g quartz and 50 g magnetite. Flotation conditions were similar to those of magnetite particle flotation alone, in order to facilitate comparison of the results of the two sets of tests.
All flotation tests were done in triplicate, and the reproducibility of the tests was good.
Results and discussion
Magnetite particle shape measurement
From each size fraction, representative samples were prepared for SEM studies, and the images were studied by the image analysis system. The results are given in Table I. These results were obtained using the image analysis system and Equations [2] to [5].
According to the grinding mechanisms in ball and rod mills, it is expected that rod mill products will have a higher elongation value and lower roundness value than ball mill products.
Table I shows that for the -250+212 µm and -106+ 75 µm size fractions, the ball mill products have a higher elongation value and lower roundness value than the rod mill products, while the for the -150+125 µm size fraction, the rod mill products have a higher elongation value and lower roundness value. Therefore, the results show that particle shape depends not only on the type of mill and grinding mechanism, but also on the mineral structure and properties (Forssberg et al., 1985). Table I and Figure 2 show that the ball mill products in the larger size fractions have higher roundness value and lower elongation ratio value, while in rod mill products, the -150+125 (im size fraction has the maximum elongation ratio value and minimum roundness value.

Flotation tests
Flotation of magnetite alone
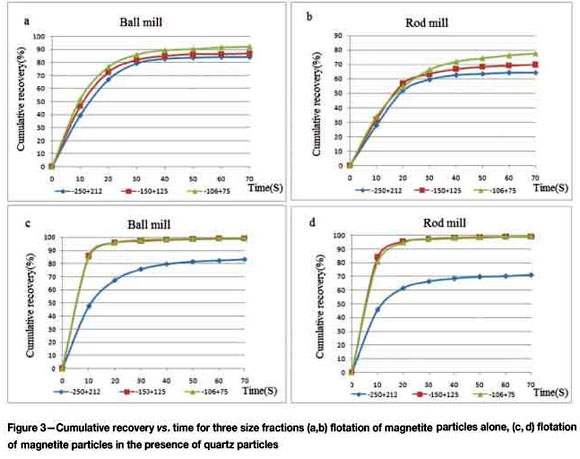
Tables II and III show the results of magnetite flotation tests for the -250+212 (µm and -106+75 µm size fractions, respectively. The flotation kinetic constants for the -250+ 212 µm size fraction of the ball and rod mill products are 4.44 (min-1) and 4.24 (min-1), respectively. The flotation kinetic constants for the -106+75 (µm size fraction of the ball and rod mill products are 5.16 (min-1) and 4.61 (min-1), respectively. It can be seen from Tables I-III that in these size fractions, ball mill products that have a higher elongation value and lower roundness value than rod mill products, have higher flotation kinetic constants. Therefore, particles with a higher elongation ratio and lower roundness have faster flotation kinetics.
The flotation kinetics for the -150+125 µm size fraction of the ball and rod mill products are 5.063 (min-1) and 4.372 (min-1), respectively. Therefore, in this size fraction, the flotation kinetic constant of the ball mill products is higher than that of the rod mill products.
It can be seen from Table I that in this size fraction, the elongation values of magnetite particles for the ball and rod mill products are 1.457 and 1.538 respectively, and the roundness values are 0.949 and 0.933 respectively. Therefore, in this size fraction, the elongation value for the rod mill products is higher than for the ball mill products. Even though the rod mill products have a higher elongation value than the ball mill products in this size fraction, rod mill products have a lower flotation kinetic constant. Therefore, it can be concluded that in this size fraction, the effect of other parameters, such as surface roughness, on flotation kinetics is greater than the effect of particle shape parameters (Rahimi et al., 2012a).
Magnetite flotation tests in the presence of quartz particles
Tables V and VI show results of magnetite flotation tests in the presence of quartz particles for the -250+212 µm and -106+75 µm size fractions, respectively. The flotation kinetic constants for the -250+212 µm size fraction of ball and rod mill products are 5.930 (min-1) and 4.99 (min-1) respectively, and for the -106+75 µm size fraction of ball and rod mill products, 11.78 (min-1) and 10.06 (min-1) respectively.
Tables I, V, and VI show that in these size fractions, ball mill products, which have higher elongation value and lower roundness value than rod mill products, have faster flotation kinetics.
It can be seen that for these size fractions, the products of both rod and ball milling have higher flotation kinetic constants in the presence of quartz particles. Therefore, it can be concluded that when magnetite particles are floated in the presence of another mineral, the influence of particle shape parameters on the flotation kinetic constant is increased due to particle-particle interaction.
Table VII shows the results of magnetite flotation tests in the presence of quartz particles for the -150+125 µm size fraction. The flotation kinetic constants for the ball and rod mill products are 11.357 min-1 and 11.05 min-1, respectively.
It can be seen from Table I that in this size fraction, elongation values for ball and rod mill products are 1.457 and 1.538, respectively. In this size fraction, the flotation kinetic constant of the ball mill products is higher than that of the rod mill products, even though the rod mill products have a higher elongation value than the ball mill products. It can therefore be concluded that in -the 150+125 µm size fraction, the effect of other parameters, such as surface roughness, on flotation kinetics is greater than the effect of particle shape parameters (Rahimi et al., 2012a).
The results show that the magnetite in the 150+125 um size fraction from both rod and ball milling has a higher flotation kinetic constant in the presence of quartz particles than when floated alone. Therefore, it can be concluded that when magnetite particles are floated in the presence of another mineral, the influence of particle shape parameters on the flotation kinetic constant is increased due to particle-particle interaction.
It can be concluded from this work that particles with a higher elongation value and lower roundness value have faster flotation kinetics. Furthermore, in the presence of quartz particles, the influence of particle shape on magnetite flotation is enhanced, and the flotation kinetic constant is higher than for magnetite alone.
It can be seen from Figures 3 and 4 that the elongation ratio (Ro) and flotation kinetic constants of ball mill products (for both magnetite flotation alone and in presence of quartz particles) increase with size reduction. For rod mill products, the -150+125 µm size fraction has the maximum elongation ratio (Ro). For flotation of magnetite alone, the maximum flotation kinetic constant is found in the -106+75 um size fraction, and for flotation in the presence of quartz particles, in the -150+125 µm size fraction. It can be seen from Figure 4 that in the presence of quartz particles, the flotation kinetic constant trend for ball and rod mill products follows the trend of the elongation ratio. This is due to particle-particle interaction.
Conclusions
1. In the -250+212 µm and -106+75 µm size fractions, ball mill products have higher elongation values and lower roundness values than rod mill products, while in the -150+125 µm size fraction, rod mill products have higher elongation value and lower roundness than ball mill products
2. In the flotation of magnetite particles alone and in the presence of quartz particles, the -250+212 µm and -106+ 75 µm size fractions of ball mill products, which have higher elongation values and lower roundness values than those of rod mill products, have faster flotation kinetics
3. In the flotation of magnetite particles alone and in the presence of quartz particles, even though rod mill products have higher elongation values than ball mill products in the-150+125 µm size fraction, rod mill products have a lower flotation kinetic constant. Therefore, it can be concluded that, in this size fraction, the effect of other parameters, such surface roughness, on recovery is greater than the effect of particle shape parameters
4. In magnetite flotation in the presence of quartz particles, the influence of magnetite particle shape on flotation is higher, and the flotation kinetic constant is higher, than for magnetite flotation alone.
References
Agar, G.E., Chia, J., and Requis, C.L. 1998. Flotation rate measurements to optimize an operating circuit. Minerals Engineering, vol. 11, no. 4. pp. 347-360. [ Links ]
Ahmed, M.M. 2010. Effect of comminution on particle shape and surface roughness and their relation to flotation. International Journal of Mineral Processing, vol. 94, no. 3-4. pp. 180-191. [ Links ]
Çilek, E.C. 2004. Estimation of flotation kinetic parameters by considering interactions of the operating variables. Minerals Engineering, vol. 17, no. 1. pp. 81-85. [ Links ]
Durnay, T.E. and Meloy, T.P. 1986. Particle shape effects due to crushing method and size. International Journal of Mineral Processing, vol. 16, no. 1-2. pp. 109-123. [ Links ]
Forssberg, E. and Zhai, H. 1985. Shape and surface properties of particles liberated by autogenous grinding. Scandinavian Journal of Metallurgy, vol. 14. pp. 25-32. [ Links ]
Hicyilmaz, C., Ulusoy, U., and Yekeler, M. 2004. Effects of the shape properties of talc and quartz particles on the wettability based separation processes. Applied Surface Science, vol. 233, no. 1-4. pp. 204-212. [ Links ]
Huh, C. and Mason, S.G. 1974. The flotation of axisymmetric particles at horizontal liquid interfaces. Journal of Colloid and Interface Science, vol. 47, vo. 2. pp. 271-289. [ Links ]
Klimpel, R.R. 2000. Optimizing the industrial flotation performance of sulfide minerals having some natural floatability. International Journal of Mineral Processing, vol. 58, no. 1-4. pp. 77-84. [ Links ]
Kuopanportti, H., Suorsa, T., Dahlo, O., and Niinimaki, J. 2000. A model of conditioning in the flotation of a mixture of pyrite and chalcopyrite ores. International Journal of Mineral Processing, vol. 59, no. 4. pp. 327-338. [ Links ]
Kursun, H. and Ulusoy, U. 2006. Influence of shape characteristics of talc mineral on the column flotation behavior. International Journal of Mineral Processing, vol. 78, no. 4. pp. 262-268. [ Links ]
OliveiraLIVEIRA, J.F., Saraiva, S.M., Pimenta, J.S., and Oliveira, A.P.A. 2001. Kinetic of pyrochlore flotation from Araxa mineral deposits. Minerals Engineering, vol. 14, no. 1. pp. 99-105. [ Links ]
Rahimi, M., Dehghani, F., Rezai, B., and Aslani, M.R. 2012a. Influence of the roughness and shape of quartz particles on their flotation kinetic. International Journal of Minerals, Metallurgy and Materials, vol. 19, no. 4. pp. 284-289. [ Links ]
Rahimi, M., Aslani, M.R., and Rezai, B. 2012b. Influence of surface roughness on flotation kinetic of quartz. Journal of Central South University of Technology, vol. 19, no. 5. pp. 1206-1211. [ Links ]
Ulusoy, U., Hicyilmaz, C., and Yekeler, M. 2004. Role of shape properties of calcite and barite particles on apparent hydrophobicity. Chemical Engineering and Processing, vol. 43, no. 8. pp. 1047-1053. [ Links ]
Xu, M. 1998. Modified flotation rate constant and selectivity index. Minerals Engineering, vol. 11, no. 3. pp. 271-278. [ Links ]
Paper received Apr. 2012
Revised paper received Sep. 2013
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN 2225-6253.














