Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 no.11 Johannesburg Nov. 2013
Moisture adsorption and desorption characteristics of some South African coals
Q.P. Campbell; M.D. Barnardo; J.R. Bunt
School of Chemical and Minerals Engineering, North-West University, Potchefstroom, South Africa
ABSTRACT
The high final total moisture content of fine coal after mining and processing is one of the major reasons that this resource is not extensively used in subsequent power generation, combustion, or other conversion processes. Some coal products, like export coal, may be subjected to a variety of environmental conditions during transport and storage, such as temperature and humidity. To understand the mechanisms by which moisture is attracted and held on and within fine coal particles, further information is needed regarding the processes occurring at the coal surface. In order to determine the correlation between physical coal properties and its moisture adsorption and desorption characteristics, a series of sorption experiments were conducted on various coal samples under climatically controlled conditions. Equilibrium moisture data was collected while changing the temperature and humidity. This data was correlated to coal properties such as particle size, porosity, maceral composition, and mineral content. All the coals that were studied were medium-rank bituminous coals. It was found that the best predictors for moisture adsorption and desorption were the mineral and inertinite contents.
Keywords: moisture, adsorption, desorption, fine coal, surface properties.
Introduction
South Africa produces about 250 Mt of coal annually, of which about 70 Mt are exported, and the rest used mostly for domestic power generation and synthetic fuel production (Department of Minerals and Energy, 2008). The coal reserves in the country are mainly bituminous, and are relatively low in sulphur (typically less than about 1%). Because of the nature of the mineral matter in the coal, it is difficult to beneficiate, and therefore some power station feeds can contain up to 40% mineral matter (Pinheiro, 1999). Typical of Gondwana coals, the vitrinite content can vary from 5% up to 90% for the northernmost coalfields. The other common maceral group is inertinite, while liptinite occurrence is typically less than less than 5% (Falcon and Snyman, 1986).
Investigators have stated that moisture in coal, especially fine coal, affects the product in the following ways: the net calorific value- decreases, handling becomes more difficult, and transport costs increase (Petrick, 1970). Producers spend up to R3 (about 50 US cents) per ton of dewatered coal to reduce the final moisture level to contract levels (De Korte, 2001).
The nature and mechanism of water adsorption, attachment, and desorption on coal surfaces and other coal char surfaces had been studied by many researchers over the years (Bradley and Rand, 1995; Nishino, 2001; Pendleton et al, 2002; Prinz and Littke, 2005; Qi et al., 2000). Many concentrated on the interaction of clean, homogeneous surfaces with water under controlled laboratory conditions (Arenillas et al., 2004). A variety of adsorption isotherms were determined, and the types and parameters tended to vary greatly with the coal type, rank, and source. Isotherms like the Dubinin-Astakhov Equation [1] have been used to characterize water adsorption on microporous carbons with some success (Bradley and Rand, 1995; Prinz and Littke, 2005).
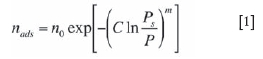
where nads is the moles of water adsorbed, Ps the saturation vapour pressure, and P the partial pressure of water. The fitted parameters of the model are no (the maximum adsorption, m (related to the pore size distribution, and C (a thermodynamic parameter related to the energy of adsorption and the temperature).
These results from this project were used to characterize the chemical and physical properties of the coal surfaces, contributing greatly to the understanding of the fundamentals of water adsorption on coal.
Other researchers found that existing isotherms did not fit very well, and empirical relationships had to be developed (McCutcheon et al., 2001).
The research described in this paper was undertaken specifically to be able to predict the water adsorption behaviour on the coal product while being stored or transported. Since the distance between the main producing coalfields in the Witbank area and the largest export terminal, at Richards Bay is about 500 km, the coal is exposed to a variety of environmental conditions over a period of days, or even weeks. Coal product samples from collieries representing three coalfields (Waterberg, Witbank, and Free State) were exposed to simulated environments and their responses correlated with some of the physical coal properties. Many coalfields are situated in areas with dry cold winters and hot rainy summers, while the coal terminals on the coast generally have high temperatures and relative humidity all year round.
Experimental
Coal characterization
Coal samples from three major South African coalfields (identified as Waterberg - GG, Witbank 4 seam - WB, and Free State - FS) were used in the investigation. Coals from the GG and WB sources are classified as medium-rank bituminous, while the FS sample was a medium-rank bituminous D coal, as determined by vitrinite random reflectance analysis (Pinheiro, 1999). These sources were chosen to ensure a variation in mineral type and content, as well as maceral composition.
Each of the three samples was split into three size fractions (-0.5 mm, +0.5 mm -1 mm, and +1 mm - 2 mm) by dry sieving, without any additional intentional size reduction, in order to ensure that the fractions closely represent the products broken during normal transport and handling.
Samples were stored in airtight containers under a controlled conditioned environment (22°C and 40% relative humidity) prior to use.
A summary of the proximate analyses and other properties of the samples as received is given in Table I, as determined using standard SABS / ISO methods. The surface characteristics of each coal sample were also determined using a mercury intrusion porosimeter, and a summary of the results is shown in Table II. Note that the porosity of the -0,5 mm fraction could not be determined due to equipment failure.
Environment chamber
A controlled environment chamber was developed to test the bulk moisture adsorption and desorption characteristics of coal. The apparatus consisted of a 0.5 m3 insulated chamber fitted with a sample holder mounted on a load cell. Air was circulated through the chamber at 0.005 m3/s by an external fan. This air could be optionally passed through an air conditioner for cooling and drying. A standard household humidifier was installed in the chamber, as well as heating elements. The on/off status of the air conditioner, humidifier, and heating elements was controlled based on the measurements of a thermocouple/humidity sensor (Delta Ohm HD8508TC150) mounted in the chamber. A multivariable control strategy was developed to decouple and control the humidity and temperature independently at specified set-points. On-line mass measurements were made while the samples were subjected to various environmental conditions. The laboratory where the tests were performed is at an altitude of 1 400 m above sea level, which meant that the average atmospheric pressure was about 850 kPa.
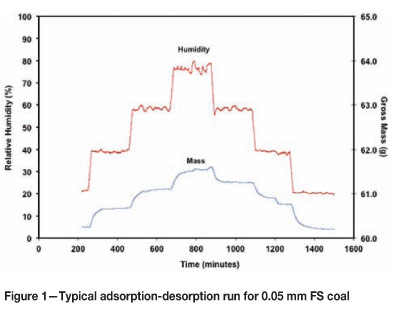
A series of experiments was conducted for each of the samples, at three different air temperatures: 15°C, 20°C, and 25°C. The experimental procedure involved placing about 50 g of sample in the chamber, and then setting the humidity set-point at 20% relative humidity (RH). While the equilibrium moisture contents of the coal samples were not determined by standard methods, the system was allowed to reach equilibrium, indicated by a constant mass reading, at this initial relative humidity level. The humidity was then increased to 40%, 60%, and 80% RH in turn, again allowing for equilibrium between steps. Thus 80% was the highest possible humidity level before water started to condense on the equipment and instruments inside the chamber. After equilibrium at 80% RH, the set-points were decreased in the same manner in steps back to 20% to record desorption responses. The time taken to complete each experiment was in the order of 36 hours, to ensure equilibrium at each humidity level. The sample was then removed from the chamber and analysed immediately for moisture content in a vacuum oven, using the SANS 5924 standard (SANS, 2009). This enabled the determination of the mass of adsorbed moisture per unit mass of coal for each intermediate equilibrium. Figure 1 shows a typical plot of the data generated during such a run.
Results and discussion
In order to quantify the adsorption and desorption parameters, attempts were made to fit the data to known isotherms normally used in these types of studies. From the literature, water adsorption on bituminous coal is usually described as following a Type II isotherm (Mahajan and Walker, 1971; Unsworth et al., 1989). We attempted to use the BET equation, but it was not possible to fit this to the data with sufficient confidence due to the limited partial pressure range of the experiments. The same applied to the Langmuir isotherm. The Dubinin-Astakhov Equation [1] fitted the desorption data fairly well over a limited range, but for adsorption, none of the equations was found to describe the data adequately.
Figure 2 shows the desorption data together with the fitted Dubinin-Astakhov Equation. The no parameter of the equation was not used to quantify the maximum adsorption for monolayer coverage, since this could not be extrapolated accurately from the data. Instead, the maximum equilibrium water adsorption (nMAX) under the highest relative humidity condition (about 80% RH) was selected to indicate the potential of a particular bulk coal to adsorb atmospheric- moisture.
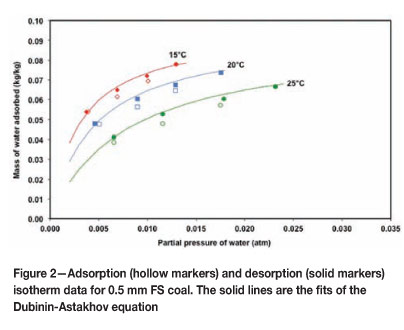
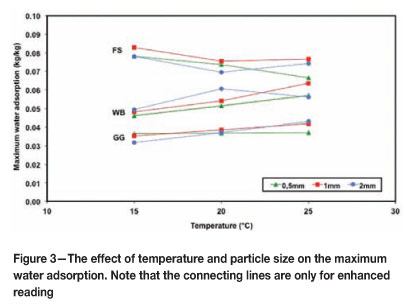
Figure 3 shows this parameter plotted against temperature for the different particle sizes. It can be seen that it is largely independent of temperature, as expected (Ruthven, 1984). The particle size also has little effect, since the adsorption is primarily dependent on the volume of the pores, and less on the surface of the particle, which is an order of magnitude smaller. Other studies (Monazam et al., 1998) have shown that the particle size dramatically influenced the rate of hydration but has no effect on the equilibrium moisture content. Any variation is probably due to incomplete liberation, and thus varying properties within the size intervals.
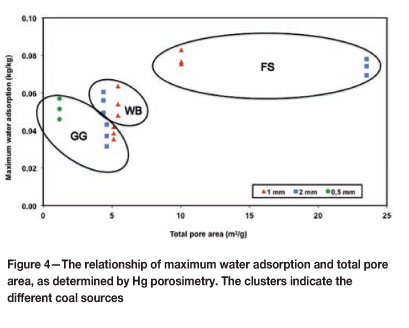
The maximum water adsorption level was then correlated with selected physical coal properties. Figure 4 shows that there is no clear relationship between nMAX and the total surface area of the pores. This finding corresponds with that of Youssef (1974), who stated that the adsorption of water is related to the surface functional groups rather than to the extent of the surface. Unsworth et al. (1989) also describe a poor correlation between these two parameters, due mainly to the size range of pores that can be measured using mercury porosimetry.
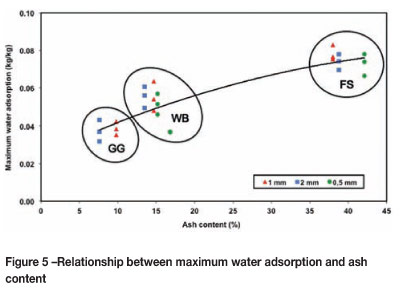
The strong effect of the mineral content on nMAX is shown in Figure 5. Since clay minerals (illite, kaolinite, and montmorillonite) form a major constituent of the mineral matter in South African coals, especially as finely disseminated syngenetic particles in the Free State coals (Falcon and Snyman, 1986), the increased water adsorption may be due to the presence of more clay minerals. This corresponds with a study on Australian coal where the montmorillonite content played a major part in water adsorption (McCutcheon and Barton, 1999). A further study is planned to investigate this for South African coals
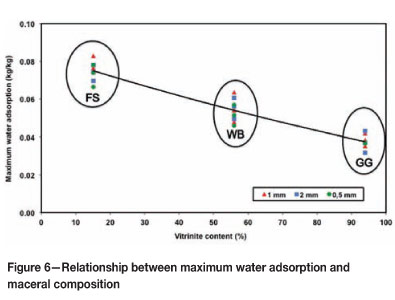
The effect of the maceral composition is illustrated by Figure 6, showing the variation of nMAXversus the vitrinite content. Inertinite contains more macroporosity (30 nm to 10 µm pore diameter) than vitrinite (Unsworth et al., 1989), and this demonstrates some agreement with higher inherent moisture. Also, inertinite tends to contain more oxygen functional groups, and thus more hydrophilic sites.
The effect of the volatile content on nMAX was also determined, and followed a similar trend to that reported by Prinz and Littke (2005), where a lower volatile content yielded higher moisture adsorption, although in that research the volatile content was used as a rank indicator. Since the definition of volatile matter is not specific to the species involved, this correlation is not considered very useful in terms of moisture adsorption.
Hysteresis between adsorption and desorption was found in all cases, and is in agreement with most other research findings. Various explanations for hysteresis are given by Mahajan and Walker (1971) McCutcheon et al (2003)- Mitropoulos et al. (1996) and Tarasevich (2001), where it is stated that low-pressure hysteresis should correlate with oxygen content, while high-pressure hysteresis is associated with pores having restricted openings and referred to as 'ink-bottle' type pores. However, no relation between the degree of hysteresis and the physical coal properties under discussion here could be found.
Conclusions
The understanding and description of the mechanisms of moisture adsorption on coal is as complex as the coal itself. While many researchers have published their findings using specific types of coal, or even clean carbon surfaces, under very specific conditions, our research was conducted using bulk coal samples that included great variability. The conditions were chosen to emulate the environments that most coals will be subjected to during normal transport and storage. While more detailed investigations are required, these results will contribute to predicting adsorption and desorption responses for the particular coals studied.
Acknowledgements
The authors wish to thank the Coaltech 2020 Research Programme and their member organizations for financial support and permission to publish the results of this investigation. This work is based on the research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa. Any opinion, finding, or conclusion or recommendation expressed in this material is that of the author(s) and the NRF does not accept any liability in this regard.
References
Arenillas, A., Pevida, C., Rubiera, F., Palacios, J.M., Navarrete, R., Denoyel, R., Rouquerol, J., and Pis, J.J. 2004. Surface characterisation of synthetic coal chars made from model compounds. Carbon, vol. 42. pp. 1345-1350. [ Links ]
Bradley, R.H. and Rand, B. 1995. On the physical adsorption of vapors by microporous carbons. Journal of Colloid and Interface Science, vol. 169. pp. 168-176. [ Links ]
De Korte, G.J. 2001. Dewatering and drying of fine coal: Survey of dewatering costs. Report: Coaltech 2020 project no. Y3675. Division of Mining Technology, CSIR, Pretoria, South Africa. [ Links ]
Department of Minerals and Energy. 2008. South Africa's Mineral Industry 2007/2008. Directorate: Mineral Economics, Pretoria, South Africa. [ Links ]
Falcon, R.M.S. and Snyman, C.P. 1986. An introduction to coal petrography: Atlas of petrographic constituents in the bituminous coals of Southern Africa. Geological Society of South Africa, Johannesburg, South Africa. [ Links ]
Mahajan, O.P. and Walker, P.L. 1971. Water adsorption on coals. Fuel, vol. 50. pp. 308-317. [ Links ]
McCutcheon, A.L. and Barton, W.A. 1999. Contribution of mineral matter to water associated with bituminous coals. Energy and Fuels, vol. 13. pp. 160-165. [ Links ]
McCutcheon, A.L., Barton, W.A., and Wilson, M.A. 2001. Kinetics of water adsorption/desorption on bituminous coals. Energy and Fuels, vol. 15. pp. 1387-1395. [ Links ]
McCutcheon, A.L., Barton, W.A., and Wilson, M.A. 2003. Characterization of water adsorbed on bituminous coals. Energy and Fuels, vol. 17. pp. 107-112. [ Links ]
Mitropoulos, A.C., Haynes, J.M., Richardson, R.M., Stetiotis, T.A., Stubos, A.K., and Kanellopoulos, N.K. 1996. Water adsorption and small angle X-ray scattering studies on the effect of coal thermal treatment. Carbon, vol. 34. pp. 775-781. [ Links ]
Monazam, E.R., Shadle, L.J., Evans, R., and Schoeder, K. 1998. Water adsorption and desorption by coals and chars. Energy and Fuels, vol. 12. pp. 1299-1304. [ Links ]
Nishino, J. 2001. Adsorption of water vapor and carbon dioxide at carboxylic functional groups on the surface of coal. Fuel, vol. 80. pp. 757-764. [ Links ]
Pendleton, P., Wu, S.H., and Badalyan, A. 2002. Activated carbon oxygen- content influence on water and surfactant adsorption. Journal of Colloid and Interface Science, vol. 246. pp. 235-40. [ Links ]
Petrick, A.J. 1969. Moisture in coal: It's occurence, it's implications and some of the problems met in practice. Report no. 16. Fuel Research Institute of South Africa, Pretoria, South Africa. [ Links ]
Pinheiro, H.J. (ed.) 1999. Bulletin 113 - Analyses of coal product samples of South African collieries 1998 - 1999. South African Bureau of Standards, Coal and Mineral Services, Pretoria, South Africa. [ Links ]
Prinz, D. and Littke, R. 2005. Development of the micro- and ultramicroporous structure of coals with rank as deduced from the accessibility to water. Fuel, vol. 84, pp. 1645-1652. [ Links ]
Qi, S., Hay, K.J., Rood, M.J., and Cal, M.P. 2000. Equilibrium and heat of adsorption for water vapor and activated carbon. Journal of Environmental Engineering, vol. 126. pp. 267-271. [ Links ]
Ruthven, D.M. 1984. Principles of adsorption and desorption processes. John Wiley and Sons, New York. [ Links ]
Sans (South African National Standards). 2009. Moisture content of coal samples intended for general analysis (vacuum-oven method). Pretoria: SABS, Standards Division. (SANS 5924). [ Links ]
Tarasevich, Y.I. 2001. Porous structure and adsorption properties of natural porous coal. Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 176. pp. 267-272. [ Links ]
Unsworth, J.F., FowlerO, C.S., and Jones, L.F. 1989. Moisture in coal. Fuel, vol. 68. pp. 18-26. [ Links ]
Youssef, A.M. 1974. Moisture sorption in relation to some characteristics of coal. Carbon, vol. 12. pp. 433-438. [ Links ] ♦
Paper received Jan. 2013
Revised paper received Apr. 2013
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN 2225-6253.














