Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 no.9 Johannesburg ene. 2013
PRESIDENTIAL ADDRESS
The role of metallurgy in enhancing beneficiation in the South African mining industry
M. Dworzanowski*
SYNOPSIS
Beneficiation, in the context of this paper, has two distinct definitions. From an economic perspective, beneficiation relates to adding value to a mined raw material. From a metallurgy perspective, beneficiation relates to processes used to upgrade the mined raw material. Clearly then, 'economic beneficiation' is dependent on 'metallurgical beneficiation'. South Africa possesses the world's largest mineral resources by value and is a significant global producer of many mined commodities. Significant metallurgical beneficiation of ores does occur in South Africa.
The role of metallurgy in economic beneficiation will be examined in terms of current practice and the potential for expansion. South Africa's main mining commodities will be covered - namely gold, platinum, coal, iron ore, manganese, chromium, vanadium, copper, nickel, titanium, zirconium, uranium, and diamonds.
Introduction
From a metallurgy perspective, beneficiation relates to processes used to upgrade the mined raw material or 'run of mine' (ROM) ore. The fundamental objective of the application of metallurgy is to produce the materials required for fabrication and manufacturing. In the case of coal the objective is to produce a fuel and/or a reductant.
The mining value chain essentially describes the path from discovering a mineral deposit to producing semi-fabricated products. This value chain has five components, which are described in Figure 1.
Metallurgy is also involved in the production of semi-fabricated components, since this relies heavily on physical metallurgical input.
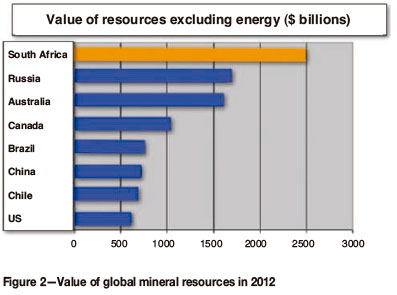
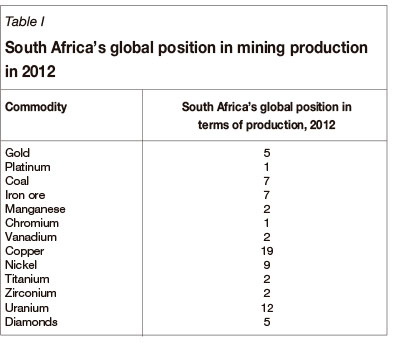
South Africa possesses the world's largest mineral resources by value, as shown in Figure 2. It is also a significant global producer of many mined commodities, as shown in Table I. The role of metallurgy in the mining value chain will be examined in terms of what is practised currently and the potential to expand. The optimum degree of metallurgical beneficiation is highly dependent on economic factors such as cost (capital and operating), appropriate skills availability, production scale, and market considerations.
Definitions
It is important to start with clear definitions around metallurgy and beneficiation, given their close links.
A practical definition of extractive metallurgy is the extraction of minerals or metals from their ores by a combination of liberation, separation, and transformation. The definitions of liberation, separation, and transformation can be given as follows:
> Liberation-preparation of the ore for separating valuable minerals from waste or 'gangue' minerals, achieved by reducing the particle size of the ore, for example, crushing and grinding
> Separation-achieved by taking advantage of differences in physical properties between the valuable minerals and the waste or gangue minerals in the ore. Examples of these properties are:
- Density (light or heavy), which is covered by dense medium separation and gravity concentration
- Magnetic or non-magnetic, which is covered by magnetic separation
- Mineral surface properties (hydrophobic or hydrophilic), which is covered by flotation
> Transformation-conversion of the valuable minerals to metals or other saleable products. Achieved by treating the valuable minerals that have been liberated and separated using thermal and/or chemical processes.
The practical definitions of physical metallurgy, 'economic beneficiation', and beneficiation can be given as follows:
> Physical metallurgy is the transformation of metal products into alloys and/or semi-fabricated products such as wire, coil, plate, pipe, etc.
> 'Economic beneficiation' is the transformation of mined ore into a higher value product that can be consumed locally or exported
> Beneficiation as related to metallurgy is the treatment of raw material (such as iron ore) to improve its physical or chemical properties, especially in preparation for smelting.
Phases of metallurgical processing
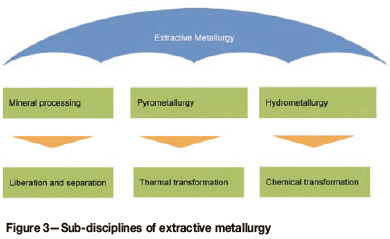
The application of extractive metallurgy through the steps of liberation, separation. and transformation can be covered by the three sub-disciplines of extractive metallurgy. Mineral processing covers liberation and separation; pyrometallurgy covers transformation utilizing thermal processes; hydrometallurgy covers transformation utilizing chemical processes. The effective application of extractive metallurgy to the beneficiation of ROM ore will generally involve all three sub-disciplines - coal and diamonds being the no table exceptions, where only mineral processing is applied. Figure 3 illustrates the subdivisions of extractive metallurgy.
The metal products resulting from the application of extractive metallurgy will then proceed to the physical metallurgy processes. These processes can start with the production of alloys such as stainless steel, brass, bronze, etc., followed by semi-fabrication processes. Alternatively, the metal products are taken straight to semi-fabrication processes, usually via melting. Semi-fabrication processes cover the production of plate, rod, wire, pipe, etc., via casting, drawing, forging, rolling, annealing, etc. Figure 4 illustrates the wide range of physical metallurgy processes used in the production of stainless steel.
Purity of product related to complexity of metallurgical processing
The treatment of ROM ore by metallurgical processes to produce feed for alloying or semi-fabrication involves a series of steps whereby the concentration of the metal of value increases in each successive intermediate product. This is not just a case of upgrading, but also of meeting very particular chemical specifications. More often than not it is the removal of the last traces of certain impurities that introduces significant complexities to the metallurgical processing required.
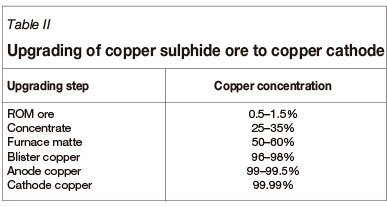
A good example of the requirement for numerous upgrading steps is the production of copper. Most copper globally is produced from copper sulphide ore, South Africa being no exception. Table II shows the steps required and how the concentration of copper in the intermediate products increases.
The ROM ore is crushed, milled, and undergoes flotation to produce a concentrate. At this point the concentrate can be sold for toll smelting and refining. The concentrate is smelted in a reverberatory or flash furnace to a furnace matte. The furnace matte is then treated in Pierce Smith converters to produce blister copper. The blister copper can be sold for further treatment, but this is not common practice. The blister copper is fire refined in anode furnaces, which produce copper cast into anodes. The anodes can be sold for refining, but this is also not common practice. The copper anodes are refined in electrolytic refineries to produce copper cathode.
Current intensity of metallurgical processing in South Africa
Gold
The ROM ore undergoes crushing and milling before the gold is dissolved using cyanide. The gold is extracted from the cyanide solution using activated carbon. The gold is removed from the activated carbon into a solution. This solution undergoes electrowinning to deposit the gold onto steel wool, which is then smelted into an impure gold bar. These impure bars are sent to the Rand Refinery for final treatment to produce pure gold. This pure gold is suitable for alloying, semi-fabrication, and fabrication.
Platinum
The ROM ore undergoes crushing, milling, and flotation to produce a concentrate. This concentrate is smelted to produce a furnace matte, which in turn undergoes a converting process to produce a converter matte. At this point the converter matte reports to a base metals refinery. All the major platinum producers have differing flow sheets in their base metals refineries, but they all produce nickel, copper, and cobalt by-products. After the removal of base metals, the remaining material reports to a precious metals refinery. In South Africa there are three precious metals refineries, all utilizing different flow sheets. All three refineries produce pure platinum as well as the platinum group metals byproducts of palladium, rhodium, ruthenium, and iridium. Pure gold is also produced. Except for the cobalt and some of the nickel, all the other metals produced by the base metals and precious metals refineries can be used for alloying, semi-fabrication, and fabrication.
Coal
Most ROM coal is crushed and then processed in dense medium separation (DMS) plants. In some cases gravity concentration and flotation are also used to increase the product yield. Low-quality thermal coal is produced for Eskom, while high-quality thermal coal is exported. Currently very little coking coal is produced in South Africa -ArcelorMittal South Africa has to import most of its coking coal requirements.
Iron ore
Some iron ore requires only crushing and no further processing. This is referred to as 'direct shipping ore' (DSO). Most South African iron ore does require processing, which involves DMS or gravity concentration, usually jigging. ArcelorMittal South Africa uses lump iron ore (-25 mm + 8 mm) in their blast furnaces, and fine iron ore (-8 mm + 0.2 mm) is agglomerated in sinter plants before feeding to the blast furnaces. The blast furnaces produce pig iron, which is then converted to steel in basic oxygen furnaces (BOF). The steel produced is then transformed into numerous semi-fabricated products. It is also used for alloying to produce speciality steels, which in turn are transformed into numerous semi-fabricated products.
Scaw Metals produces pig iron by the direct reduction of lump iron ore in long rotary kilns, using coal as the reductant. The pig iron is melted and converted to steel, which is in turn converted to numerous semi-fabricated products.
Highveld Steel & Vanadium mines vanadium-rich magnetite which is crushed and then pre-reduced, in long rotary kilns, followed by smelting in electric furnaces. The vanadium content of the pig iron is removed before it is converted to steel. The steel is then converted to numerous semi-fabricated products.
Manganese
Manganese ROM ore undergoes crushing, followed mainly by DMS and gravity concentration. The manganese-rich product is mainly smelted in electric furnaces to different grades of ferromanganese. Sometimes smelting is preceded by sintering. The ferromanganese is used for alloying with steel. A small amount of the manganese-rich product undergoes chemical transformation to produce manganese metal and battery-grade manganese dioxide.
Chromium
Chromite ROM ore undergoes crushing, followed mainly by DMS and gravity concentration. The chromium-rich product is mainly smelted in electric furnaces to different grades of ferrochromium. Sometimes this is preceded by pelletizing of the chromium-rich product. The ferrochromium is used as the main ingredient for stainless steel production. Some of the chromium-rich product is sold as a concentrate for foundry sand, for feedstock for chromium chemicals production, for feedstock for chromium metal production, and as an ingredient in the manufacture of refractories.
Vanadium
Vanadium is extracted from vanadium-bearing magnetite. The ROM ore is crushed, milled, and then undergoes magnetic separation to produce a concentrate. This concentrate is roasted with sodium carbonate in a long rotary kiln to make the vanadium soluble. A chemical transformation process produces vanadium pentoxide. This is used as a catalyst in sulphuric acid plants or is transformed into ferrovanadium, which is used for alloying with steel.
Copper
The ROM ore is crushed, milled, and undergoes flotation to produce a concentrate, which is smelted to a furnace matte. The furnace matte is converted to produce blister copper, which is fire refined and cast into anodes. The copper anodes are refined in an electrolytic refinery to produce copper cathode. Copper cathode is also produced from the base metals refineries of the major platinum producers. The copper cathode is used to produce semi-fabricated products.
Nickel
Most of the nickel produced in South Africa is derived from the base metals refineries of the major platinum producers. The nickel is produced in the form of nickel sulphate or nickel metal (cathode or briquettes). Most of the nickel metal is used as the nickel component in the alloying process for stainless steel production.
Titanium
In South Africa, titanium is sourced from mineral sands deposits. The ROM material is processed via numerous stages of gravity concentration, magnetic separation, and electrostatic separation to produce ilmenite and rutile. The ilmenite is smelted in electric furnaces to pig iron, which is used mainly for steel production, and titanium slag, feedstock for the production of titanium pigments. The rutile is a feedstock for titanium metal production.
Zirconium
In South Africa, zirconium is sourced mainly from mineral sands deposits. The ROM material is processed via numerous stages of gravity concentration, magnetic separation and electrostatic separation. The zirconium is produced as a zircon product, which is a feedstock for ceramics production and for zirconium metal production.
Uranium
In South Africa, uranium is a by-product of gold production from the Witwatersrand Basin. Uranium can be extracted either before or after gold extraction, depending on the flow sheet preference as a result of differences in the ROM ore. After crushing and milling the uranium is dissolved by leaching with sulphuric acid. The uranium is recovered from the solution by solvent extraction, and from this concentrated solution the uranium is precipitated as ammonium diuranate or 'yellowcake'. The yellowcake is converted to uranium oxide, which is used as feedstock for the nuclear applications of uranium.
Diamonds
Most of the diamonds production in South Africa is based on extraction from kimberlite pipes. The ROM ore is crushed and scrubbed. A DMS process produces a diamond concentrate, which then undergoes numerous steps of screening, magnetic separation, and X-ray sorting. Sometimes chemical cleaning is also required. This results in a final concentrate that is hand-sorted into the different categories of diamonds.
Potential for increasing the role of metallurgy in South Africa
Iron ore
Iron ore produced in South Africa includes haematite-based lump (-25 mm +8 mm) and fine (-8 mm +0.2 mm) products. These products can be utilized by the local iron and steel industry. However, future increases in iron ore production in South Africa will involve a good deal of concentrate production, which is -200 µm, and will be based on both haematite and magnetite. Currently there are no metallurgical facilities that can accommodate concentrate and convert it into products suitable for the local iron and steel industry. There are two approaches by which this can be done.
The first approach would be agglomeration of the concentrate into pellets, which can then be utilized in blast furnaces, in place of or in addition to lump iron ore. Pellets of a high chemical quality can be utilized in direct reduction kilns. Haematite- and magnetite-based iron ore concentrates or blends of the two can be used as pelletizing plant feed. The viability of such an approach would be dependent on sufficient scale and acceptable capital and operating costs.
The second approach would be direct smelting of the concentrate to pig iron. Globally there are many technology developments around the smelting of iron ore concentrate, but currently no single technology has established itself as the benchmark leader. The application of these technologies is being evaluated in South Africa, and the one technology that is close to commercialization in South Africa is the Iron Minerals Beneficiation Services (IMBS) process. This will be applied to magnetite concentrate produced at Palabora Mining Company. The concentrate will be mixed with thermal coal reductant and reacted in an externally heated rotary kiln. Pig iron in the form of powder, briquettes, or granules can be produced. Figure 5 presents the IMBS flow sheet. Whether this will be the optimum technology for South Africa remains to be seen.
Chromium
Although South Africa is the world's largest producer of ferrochrome, there is currently very limited production of chromium chemicals and no production of chromium metal. The feedstocks for these products are available and the metallurgical technology is well established globally. Chemical-grade chromite concentrate is roasted with sodium carbonate in a rotary kiln to make the chromium soluble. Leaching of the calcine with water produces sodium chromate, which is the precursor for all the other commercial chromium chemicals. Metallurgical-grade chromite concentrate is leached with sulphuric acid followed by selective crystallization to produce chromium sulphate, from which chromium metal is produced by electrowinning. The economic potential for producing chromium chemicals on a larger scale and the production of chromium metal should be examined.
Titanium
Figure 6 presents a titanium beneficiation matrix. South Africa produces rutile and ilmenite as well as titanium slag. However, there is little titanium pigment production and no titanium sponge production.
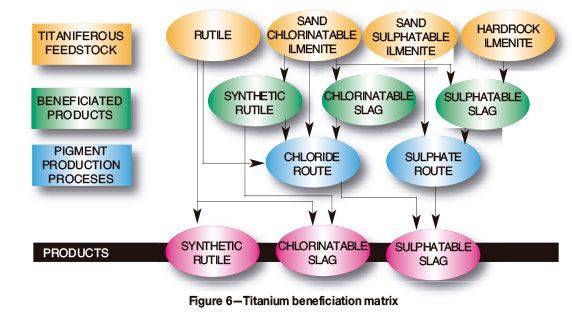
The metallurgical process for titanium pigment production is well established globally. The titanium slag is reacted with carbon and chlorine at high temperature to produce titanium tetrachloride. This is then decomposed to titanium dioxide, which is the basis for titanium pigment. The economic potential for producing titanium pigment on a larger scale in South Africa should be examined.
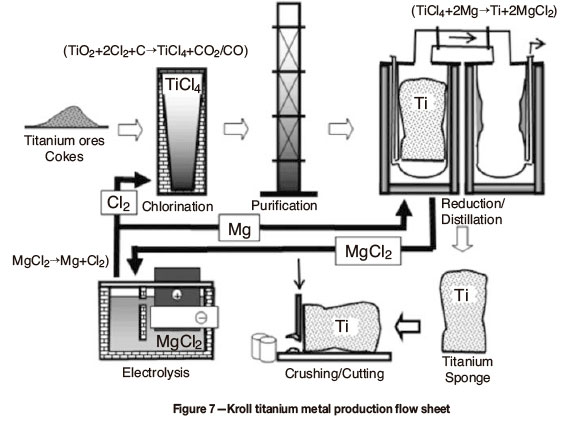
For titanium metal production, rutile is reacted with carbon and chlorine at high temperature to produce titanium tetrachloride. This is then reacted with magnesium metal to produce titanium metal sponge, which can be melted and cast into slabs and ingots. From these semi-fabricated titanium products can be derived. The current production route, known as the Kroll process, is not efficient and results in high production costs. Alternative process routes are therefore being examined in South Africa. It must be noted that numerous previous attempts at developing alternative economic processes have not been successful. The cost issue includes not only the production of the metal but also the cost of producing semi-fabricated products from the metal. Figure 7 presents the Kroll titanium metal production flow sheet.
Conclusions
South Africa possesses the world's largest mineral resources by value and is a significant global producer of many mined commodities. The current role of metallurgy in the South African mining industry is considerable, and the same can be said in terms of the role of metallurgy in economic beneficiation. The application of metallurgical processes in South Africa has established a platform for a wide variety of products that can be utilized in alloying and semi-fabrication.
The application of metallurgical processes in the production of gold, platinum, coal, iron ore, manganese, chromium, vanadium, copper, nickel, titanium, zirconium, uranium, and diamonds to a very large extent meets South Africa's requirements for alloying and semi-fabrication products (Table III).
Although Table III reflects the significant role of metallurgy in South Africa, there are always opportunities for further expansion and three examples have been highlighted:
> The current utilization of iron ore in South Africa for steel production is well established. To maintain this into the future will require developing the utilization of iron ore concentrate, since this will assume a greater significance in the near future
> The utilization of chromium resources can be expanded into the production of chromium chemicals and chromium metal
> Titanium metal has potential globally. South Africa is not currently producing titanium metal, as current commercial processes are not economic in this application. There is therefore a need to develop appropriate new technologies to enable economic production of suitable quality titanium metal and this is receiving attention in South Africa. More emphasis and funding should, however, be applied in this area. Implementation should be in conjunction with titanium pigment production, since this is closely linked from a metallurgical process point of view.
References
Anameric, B. and Komar Kawatra, S. 2009. Direct iron smelting reduction processes. Mineral Processing and Extractive Metallurgy Review, vol. 30. pp. 1-51. [ Links ]
Coetzee, C.B. (ed.). 1985. Mineral Resources of the Republic of South Africa. 5th edition. Council for Geoscience, Pretoria. [ Links ]
Dawson, M.F. and Edwards, R.I. 1984. An alternative route for the production of chromium chemicals from chromite. Proceedings of MINTEK 50, International Conference on Mineral Science & Technology, Randburg, South Africa, March 26-30. [ Links ]
Griffiths, J. 1989. South Africa's minerals, diversity in adversity. Industrial Minerals,. August 1989. [ Links ]
Leeuw, P.J.K. 2012. A linkage model for the South African mineral sector: a plausible option. MSc thesis, University of the Witwatersrand. [ Links ]
Van Vuuren, D.S. Titanium - an opportunity and challenge for South Africa. 7th International Heavy Minerals Conference 'What next?', Durban, South Africa, 20-23 September 2009. Symposium Series S57. Southern African Institute of Mining and Metallurgy, Johannesburg, 2009. [ Links ] ♦
* Anglo American Mining & Technology Technical Solutions - Research, Johannesburg, South Africa.
© The Southern African Institute of Mining and Metallurgy, 2013. SA ISSN 2225-6253. Address presented at the Annual General Meeting on 22 August 2013.














