Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 n.8 Johannesburg Jan. 2013
GENERAL PAPERS
The manganese ferroalloys industry in southern Africa
J.D. SteenkampI; J. BassonII
IMulti Facet Consultancy, Pretoria
IIPyrotek Consulting, Pretoria, South Africa
ABSTRACT
Southern Africa has a history rich in the pyrometallurgical processing of ores. Two of the alloys produced are high-carbon ferromanganese (74-83 per cent Mn) and silicomanganese (59-67 per cent Mn and 14-31 per cent Si). The largest land-based manganese ore deposit in the world is based in the Northern Cape Province of South Africa. In 2010, 6.3 Mt of ore from the deposit was exported, mainly to China, Norway, Japan, and India, and an estimated 1.1 Mt beneficiated locally to produce manganese ferroalloys. In southern Africa the producers of manganese ferroalloys are Metalloys and Assmang, and of silicomanganese Transalloys, Mogale Alloys, and Match Corporation. Submerged arc furnace technology is utilized in the production of high-carbon ferromanganese (HCFeMn) and silicoman-ganese, applying the discard slag practice in both instances. The carbon content of HCFeMn is reduced using converter technology and that of SiMn using the Perrin process. Ninety per cent of the world's manganese ferroalloys production is consumed by the steel industry. The consumption (and production) of manganese ferroalloys therefore closely follows worldwide steel production. From 2001 to 2010, the doubling of the worldwide production of manganese ferroalloys can be attributed to an increase in production capacity in Asia and Oceania, with the largest increase in the production of SiMn. Manganese ferroalloys produced in Asia and Oceania are mainly for the local market, with North America, Europe, Russia, and Turkey the major markets for alloys from southern Africa. The major challenges faced by the southern African manganese ferroalloy producers are increasing electricity tariffs and productivity of labour, which require a concerted effort by all parties involved to achieve a satisfactory solution.
Keywords: manganese ferroalloys, ferromanganese, silicomanganese, southern Africa, South Africa, manganese ore, high-carbon ferromanganese, refined ferromanganese, refined silicomanganese.
Introduction
Southern Africa is endowed with a large variety of minerals concentrated in commercially exploitable ore deposits. The historically low cost of electrical energy and labour, and the vision of pioneers such as the late Dr H.J. van der Bijl (Basson, Curr, and Gericke, 2007; SAAE, undated), led to the development of a strong pyrometallurgical industry. By 2013, 18 different types of commodities were produced at more than 60 plants throughout the region. Two of these commodities are the manganese ferroalloys ferromanganese and silicoman-ganese (Jones, 2010), produced from the largest concentration of land-based manganese in the world (van Averbeke, 2005).
This paper presents an overview of the southern African manganese ferroalloys industry, including the location and typical mineral composition of the deposits, the alloy grades produced and the production methods applied; and the role players, their products, plant locations, and installed capacities. From a global perspective, the tonnages of manganese ferroalloys produced or imported over the ten-year period 2001-2010 shows the size and potential of the industry. The paper concludes with a discussion of the challenges faced by the manganese ferroalloys industry in southern Africa and ways in which the challenges can be addressed.
Background
Manganese is the 12th most abundant element in the Earth's crust, with an average concentration of 0.1 per cent. It is the 4th most abundant metal in commercial use. In nature, manganese is found in the form of oxides, carbonates, and silicates. Manganese was recognized and isolated as a separate chemical element in 1774 by Carl Wilhelm Scheele and Johan Gottlieb Gahn (Encyclopaedia Britannica, 2012a). The Spartans, in ancient Greece, used manganese in their steel weapons to give superior strength. The Romans and Egyptians used manganese compounds to remove or add colour to their glass during glassmaking (Olsen, Tangstad, and Lindstad, 2007), as is still done today (Reisfeld, 2001). The Chinese used it in glazes to give their pottery an aubergine colour (Encyclopaedia Britannica, 2012b). The first commercial use of manganese during the industrial revolution was as the laboratory reagent potassium permanganate (KMnO4), which was produced for the first time by Johann Glauber in 1659 (Clegg, 2013). Manganese dioxide (MnO2) was used in the manufacture of chlorine in the mid-18th century (Encyclopaedia Britannica, 2013).
The major breakthrough for manganese came when Sir Henry Bessemer used manganese as a deoxidizing agent in the steelmaking process he developed in 1860 that bore his name. He added spiegeleisen - pig iron containing high levels (10-20 per cent) of manganese and produced in blast furnaces - after the oxygen blow to remove oxygen from the steel and add manganese and carbon. The idea was patented by Robert Mushet. In 1866 Siemens patented the use of ferromanganese in steelmaking to control the levels of sulphur and phosphorous. This paved the way not only for modern steelmaking, but also for the modern production of manganese ferroalloys (Olsen, Tangstad, and Lindstad, 2007).
Commercial production of ferromanganese containing 65 per cent Mn, started in France in 1875, using blast furnace technology (Downing, 2012). From 1890 ferromanganese could also be produced by submerged arc furnace (SAF) (Downing, 2012), but for the first half of the 20th century all ferromanganese was produced in blast furnaces (Olsen, Tangstad, and Lindstad, 2007). Blast furnaces use 4-6 times as much coke per ton of ferromanganese produced as SAFs, so due to the high cost and scarcity of coke, and the relative high capital investment required to build a blast furnace, electric arc furnaces started to replace blast furnace technology in the second half of the 20th century. By 2005 approximately three-quarters of the world production was by SAF and the remainder by blast furnace (Olsen, Tangstad, and Lindstad, 2007).
Ore deposits
The largest concentration of land-based manganese ever to have formed on Earth - constituting a reserve base of 4 billion tons (80 per cent of world reserves) - is found in the Kalahari Basin, 60 km north-northwest of Kuruman in the Northern Cape Province of South Africa (van Averbeke, 2005). The Kalahari deposit (99 per cent of South Africa's manganese reserve) covers 15 km x 35 km in surface area and is 2200-2300 million years old. The deposit consists of two main types of ores: Mamatwan type, which contains 2038 per cent Mn, and Wessels type (3 per cent of the total orebody), which contains 45-60 per cent Mn (van Averbeke, 2005; Cairncross, Beukes, and Gutzmer, 1997). The location of the Kalahari Manganese Field and the distribution of the two types of ore in the orebody are indicated in Figure 1.
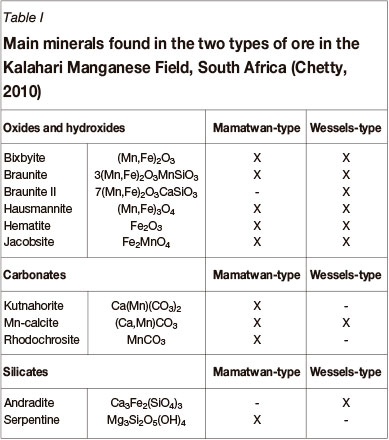
The main minerals found in each type of ore are listed in Table I (Chetty, 2010). The Mamatwan-type ore is rich in oxides, hydroxides, carbonates, and silicates, while the Wessels-type ore - a very complex ore - does not contain significant amounts of carbonates.
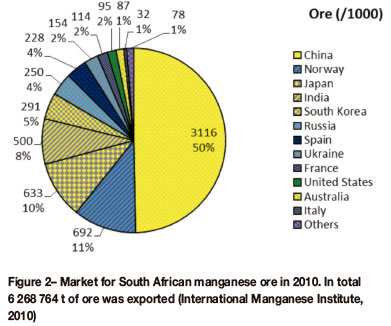
Ore from South Africa is not only beneficiated locally but also exported to international markets. Figure 2 shows the market distribution for South African ore in 2010. In total, 6.3 Mt of ore was exported, with China, Norway, and Japan being the top three importers of South African ore (International Manganese Institute, 2010). It is estimated that 1.1 Mt of ore was beneficiated to high-carbon ferroman-ganese and silicomanganese in the same year.
No detailed information on the Zambian ore deposits was found in the open literature, but ore exported from Zambia in 2010 was 76 661 t, 81 per cent of which went to China (International Manganese Institute, 2010).
Manganese ferroalloys
Manganese ferroalloys produced commercially can be divided into the following four categories (Olsen, Tangstad, and Lindstad, 2007), which are listed together with their typical chemical compositions in Table II:
High-carbon ferromanganese (HCFeMn)
Refined ferromanganese(MCFeMn)
Silicomanganese (SiMn)
Low-carbon silicomanganese (LCSiMn).

The major commercial use of manganese ferroalloys is in steelmaking. Ninety per cent of the manganese ferroalloys produced is used in steelmaking at a consumption of typically 7.5 kg Mn per ton of steel (Olsen, Tangstad, and Lindstad, 2007). The graph in Figure 3 reflects the world's real unit consumption of manganese ferroalloys in kilograms per metric ton of steel from 2001-2010 (International Manganese Institute, 2010). For the five years 2006-2010, manganese ferroalloy consumption was closer to 10 kg Mn per ton of steel.

In modern steelmaking, manganese is added to steel in order to (Olsen, Tangstad, and Lindstad, 2007; Olsen and Tangstad, 2004):
Deoxidize the steel i.e. remove oxygen from the steel as MnO, although silicon and aluminium are stronger deoxidizers. Silicon deoxidizes steel and manganese enhances the effect of silicon deoxidation by forming stable manganese silicates and aluminates
Desulphurize the steel i.e. remove sulphur from the steel as MnS
Control the morphology of the sulphides
Influence the strength, toughness, and hardness of the steel.
In steelmaking SiMn is preferred over HCFeMn and FeSi as an additive because the liquid silicate product that forms during deoxidation is easier to remove from the metal than the solid SiO2 that forms when using FeSi. Standard SiMn also has lower levels of trace elements (i.e. P, C, Al, and N) than standard HCFeMn and FeSi (Olsen, Tangstad, and Lindstad, 2007). Figure 3 depicts the rise in consumption of SiMn and decrease in consumption of HCFeMn over the 10-year period. This trend is explained by the relative increase in mini-mills, as the preference for SiMn is more prevalent in the production of flat and long steel products in mini-mills, while HCFeMn is favoured by integrated steel plants. The refined grades, refined FeMn and LCFeSi, are used in steels with low carbon specifications (Olsen, Tangstad, and Lindstad, 2007).
Applications of manganese, other than in manganese ferroalloys, are:
Electrolytic manganese metal, which is used primarily in alloys of aluminium and copper. In aluminium, manganese improves the corrosion resistance (Olsen, Tangstad, and Lindstad, 2007). As alloying agent it is used in silver-copper alloys to form brazing alloys (Reti, 2001)
Non-metallic applications include (Encyclopaedia Britannica, 2012):
- Potassium permanganate (KMnO4), used as a disinfectant, deodorant, and as bleaching and analytical reagent
- Manganese sulphate(MnSO4), used as fertilzser, especially in citrus production, and as a reducing agent in the manufacture of paint and varnish driers
- Manganous oxide (MnO), used as a raw material in the production of manganous salts, additives in fertilizers, and a reagent in textile printing
- Manganous chloride (MnCl2), used as catalyst in the chlorination of organic compounds and as an additive to animal feed
- Manganese dioxide (MnO2), used in dry cell batteries, as a chemical oxidant in organic synthesis, and as a raw material in the production of chemical-grade manganous oxide.
Production processes
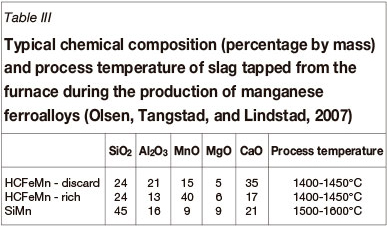
To produce manganese ferroalloys, a carbothermic reduction process is followed in which manganese-bearing minerals are reduced by solid carbon reductants to produce an alloy, slag, and off-gas (Olsen, Tangstad, and Lindstad, 2007; Habashi, 1997). From a slag chemistry perspective, three distinct processes are in operation with the typical compositions and process temperatures summarized in Table III (Olsen, Tangstad, and Lindstad, 2007):
HCFeMn production using a discard slag practice (Figure 4a)
SiMn production utilizing only ore as source of manganese (Figure 4b)
HCFeMn production using a rich ('high') slag practice, with subsequent SiMn production (Figure 5). This process is sometimes referred to as the duplex process.

In southern Africa, the first two process flow sheets are applied i.e. both HCFeMn and SiMn are produced with ore as the source of manganese. In both instances, the slag is discarded on slag dumps. Estimated figures (Kazadi, 2013) indicate that approximately 20 Mt of HCFeMn and SiMn slag has accumulated over the years on dumps in South Africa, and that on average 0.5 Mt are added to these dumps each year. Interested and affected parties are continuously seeking ways in which to reduce the size of these slag dumps in ways that are commercially viable (Kazadi, 2013; Van Reenen, Thiele, and Bergman, 2004; Reuter, Xiao, and Boin, 2004; Parker and Loveday, 1996). However, existing environmental legislation makes it very difficult to implement viable slag utilization projects.
The two main cost drivers for the production of manganese ferroalloys, apart from ore, are the price of reductant and the price of electricity (Olsen, Tangstad, and Lindstad, 2007).
Furnace technology
In southern Africa, only SAF technology is applied in the production of manganese ferroalloys. In other parts of the world, HCFeMn is also produced using blast furnace technology (Olsen, Tangstad, and Lindstad, 2007). The SAFs are typically circular with three Sõderberg electrodes in an equilateral arrangement. The furnaces can be open, semi-open, or closed, depending on the environmental regulations of each specific country. The furnaces can be stationary or rotating. Typically production furnaces carry loads of 15-45 MW, and at this size the furnaces are always stationary. In southern Africa the installed capacities range from 6 MVA to 81 MVA (estimated as 4.4 MW and 50 MW).
The SAF designs can be open (Figure 6), closed, or semi-closed. A closed furnace has a sealed roof with no or very little air ingress and the furnace off-gases are therefore not combusted. The roof of an open furnace is not sealed, and furnace off-gas is combusted on top of the furnace bed. A semi-open furnace is similar to an open furnace, but with doors installed between the furnace rim and roof to reduce air ingress and heat losses. In southern Africa all three types of furnace are in use (Habashi, 1997). The conversion between HCFeMn and SiMn production is fairly easy from a furnace design perspective (Brun, 1982), although optimal operating performance will be achieved only in a custom-designed furnace.

Manganese ferroalloy production in southern Africa
There are currently four producers of manganese ferroalloys in South Africa - Metalloys, Assmang, Transalloys, and Mogale Alloys (Basson, Curr, and Gericke, 2007), and one in Zambia - Match Corporation (Wadhavkar, 2012).
Metalloys is the largest producer of manganese ferroalloys in South Africa. Its smelter complex is based in Meyerton in Gauteng Province. The company was established originally as African Metals Corporation (Amcor) Ltd when it started producing HCFeMn in a blast furnace with a hearth diameter of 10 feet in Newcastle in 1937. In 1942, HCFeMn was produced in a 3 MVA rectangular arc furnace, with two refining furnaces for the production of MCFeMn. In 1951, operations relocated to Meyerton (Basson, Curr, and Gericke, 2007; Creamer, 2013).
Production of HCFeMn at Metalloys is estimated at 480 kt/a, (Creamer, 2013) and MCFeMn at 60 kt/a. The installed capacity included four closed SAFs, two of which are rated at 75 MVA and two at 81 MVA, for the production of HCFeMn (Basson, Curr, and Gericke, 2007; Creamer, 2012) A 30 t top-blown bottom-stirred converter is utilized for MCFeMn production (Basson, Curr, and Gericke, 2007). In 2012, Metalloys decommissioned the five semi-closed furnaces producing SiMn, and replaced the production capacity of these by a single large furnace producing HCFeMn (Creamer, 2012).
Assmang is the second largest producer of HCFeMn in South Africa, with smelter complexes based in Cato Ridge in KwaZulu-Natal and Machadodorp in Mpumalanga Province. The Cato Ridge plant was commissioned in 1957 (Basson, Curr, and Gericke, 2007). The Machadodorp works was originally commissioned as a ferrochromium (FeCr) plant, but in 2011 converted one of its furnaces to HCFeMn production, with a further two to follow.
Assmang can produce 200-240 kt/a HCFeMn at the Cato Ridge plant, and an estimated 130 kt/a HCFeMn for a two-furnace operation and 270 kt/a HCFeMn for a four-furnace operation at its Machadodorp plant. MCFeMn can be produced by top-blown bottom-stirred converter at 50 kt/a. The installed capacity includes one 24 MVA semi-open, one 24 MVA closed, two 22 MVA semi-open, and two 12 MVA closed SAFs at Cato Ridge for the production of HCFeMn. At Machadodorp, one 24 MVA and one 30 MVA semi-open SAF produce HCFeMn, with the conversion of one further 24 MVA semi-open and one 54MVA closed SAF planned. A 30 t converter is installed at Cato Ridge.
Transalloys is the largest producer of SiMn,and has its smelter complex based near eMalahleni in Mpumalanga Province. As is the case with Assmang's Machadodorp plant, Transalloys was commissioned as a FeCr plant in the 1960s but converted to a SiMn plant in 1967 (Basson, Curr, and Gericke, 2007). Currently it can produce 180 kt/a SiMn. Capacity is installed for the production of 50 kt/a MCFeMn using the Perrin process, but is currently not operational. The installed capacity include two 21 MVA open, one 23 MVA semi-open, and two 48 MVA semi-open SAFs for the production of SiMn, and two 7 MVA open arc furnaces for the production of MCFeMn (Basson, Curr, and Gericke, 2007; Transalloys, undated).
Mogale Alloys, which is based near Krugersdorp in Gauteng Province, produces primarily FeCr using DC arc furnace technology, but also produces SiMn. The company started with the production of FeCr (including LCFeCr) at its smelter complex in 1963, as the Palmiet Chrome Corporation. SiMn has been produced since the 1990s. Currently, Mogale Alloys can produce at 40 kt/a SiMn, using two 20 MVA semi-open SAFs (Basson, Curr, and Gericke, 2007).
Match Corporation is the only producer of manganese ferroalloys in Zambia. Its smelter complex is based at Luanshya in the Zambian Copperbelt. The company has two furnaces, rated at 2.4 MVA for ferrosilicon and 6.0 MVA for silicomanganese. The company started with the production of CaC2 in their 2.4 MVA furnace in 1990 and converted to FeSi production in 1994. The 6 MVA semi-closed, silicoman-ganese furnace was commissioned in July 2012. Match Corporation can produce 2 kt of FeSi and 8.5 kt of SiMn per annum (Wadhavkar, 2012).
Other projects reported in the news - but not yet executed - included a smelter at Pensulo near Serenje in the Central Province of Zambia (Zamanco Minerals, 2012) and a smelter complex at Coega in the Eastern Cape province of South Africa for Kalagadi Manganese (Kalahari Resources, undated). Planned capacity at the smelter complex at Pensulo includes production of 60 kt/a HCFeMn using three 8 MVA DC smelters and 12 kt/a LCFeMn applying aluminothermic smelting technology in which aluminium is used to reduce manganese ore. Planned capacity at the Kalagadi Manganese smelter complex is 320 kt/a HCFeMn produced by three 63 MVA or four 48 MVA closed SAFs.
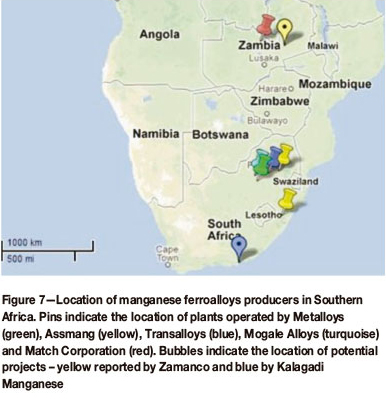
The locations of existing operations and planned projects are indicated in Figure 7. The installed capacity for southern Africa is summarized in Table IV.
World manganese production and consumption
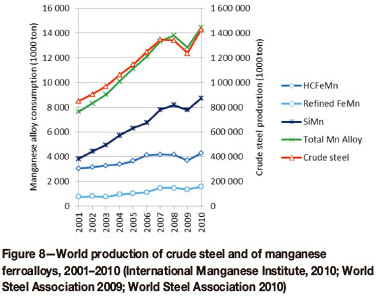
As 90 per cent of the manganese ferroalloys produced worldwide is used in steel, consumption and production closely follow the production of crude steel. For the period 2001-2010 the production of steel increased at a steady rate on an annual basis, except for a decrease in 2009 due to the economic crisis. The consumption of manganese ferroalloys followed steel production closely, as can be seen in Figure 8.
The countries where manganese ferroalloys were produced (Anon., 2010; Hearn,, Dzermejko, and Lamont, 1998) are indicated on the map in Figure 9.
In the data analysed, producers were divided into specific regions as listed in Table V. Steel production was divided into similar areas, but included more countries (International Manganese Institute, 2010; World Steel Association, 2009, 2010).

Figures 10 to 13 indicate the distribution of manganese ferroalloy production among the countries listed in Table V, comparing 2001 with 2010 (International Manganese Institute, 2010). It is interesting to note the significant increase in production in Asia, in China and India. Although the production of HCFeMn and refined FeMn alloys in the Asian countries increased, the significant increase in total Mn alloy production can be attributed primarily to the increase in SiMn production in these countries. A similar trend is observed in other regions.
This increase in production could be sustained only by an increase in the production of steel for the same period. This can be seen in Figure 14. As is the case with ferroalloy production, the overall increase in steel production can be ascribed mainly to an increase in steel production in the Asia and Oceania region.
The growth in demand for manganese ferroalloys in Asia and Oceania was met primarily by the growth in production capacity within this region. As observed in the consumption figures (Figure 15 to Figure 18), although the consumption of HCFeMn and refined FeMn alloys increased, the significant increase in total Mn alloys consumption can attributed mainly to the increase in SiMn consumption.
Challenges faced by the southern African manganese ferroalloy industry
The question arises: why, if South Africa has 80 per cent of the world's land-based manganese ore and the technology to beneficiate the ore, did it produce only 5 per cent of the world manganese ferroalloys in 2010 while exporting nearly six times more ore than was upgraded locally? The major challenges facing the ferroalloy industry, in South Africa specifically, are rising electricity tariffs and productivity of labour.
To address the problem of rising electricity tariffs, smelter operators are working on solutions to increase energy efficiency through waste heat recovery, modifying furnace designs to closed furnaces only, and power generation using off-gas. A factor not under their control, but potentially to their benefit, would be private sector involvement in reducing the basic price of electricity and exclusion of social levies from electricity tariffs. To address the problem of productivity of labour, smelter operators could work on mechanization and automation of operations, but ideally organized labour and labour legislators should be involved in efforts to increase productivity. Labour legislation amendments are also required to restore the balance of power between employers and organized labour.
Conclusions
Southern Africa has a rich history in the pyrometallurgical processing of ores. Two of the alloys produced are manganese ferroalloys (74-83 per cent Mn) and silicoman-ganese (59-67 per cent Mn and 17-31 per cent Si). South Africa has the largest land-based manganese ore deposits in the world, and not only mines and exports ore, but since 1937 has beneficiated the ore to produce manganese ferroalloys.
The large ore deposits are based in the Kalahari Basin in the Northern Cape Province of South Africa. The mineralogy of the deposits is fairly complex. Mamatwan-type ore consists of oxides, silicates, and carbonates, while Wessels-type (constituting 3 per cent of the total resources) does not contain significant amounts of carbonates. Mamatwan-type ore contains 20-38 per cent Mn, and Wessels-type contains 45-60 per cent Mn.
The manganese ferroalloys produced in southern Africa comprise different grades of ferromanganese and silicoman-ganese, which are differentiated based on their carbon content. HCFeMn contains 74-76 per cent Mn, 0.3 per cent Si, and 7.5 per cent C. Refined FeMn (MCFeMn) contains 80-83 per cent Mn, 0.6 per cent Si, and between 0.5 and 1.5 per cent C. SiMn contains 67 per cent Mn, 14-20 per cent Si, and between 1.5 and 2 per cent C. LCSiMn contains 59-6 3 per cent Mn, 26-3 1 per cent Si and between 0.05 and 0.5 per cent C.
In southern Africa both HCFeMn and standard grade SiMn are produced using only manganese ore as the major source of manganese. SAF technology is applied, and the furnaces are rated from as low as 6 MVA to as high as 81 MVA. Medium- and low-carbon ferromanganese are produced using top-blown bottom-stirred converters, and low-carbon SiMn using the Perrin process.
Five companies are actively producing manganese ferroalloys in the region. Manganese ferroalloys are produced by Metalloys and Assmang, and silicomanganese by Transalloys, Mogale Alloys, and Match Corporation. The total installed capacity for the region is 820 kt HCFeMn, 160 kt refined FeMn, and 228 kt standard grade SiMn. In South Africa, plants are situated in the Gauteng, Mpumalanga, and KwaZulu-Natal provinces, and in Zambia on the Copperbelt.
The market for manganese ferroalloys is strongly driven by the demand for steel. Manganese ferroalloys are produced commercially in 23 countries, with countries in Asia and Oceania, especially China and India, producing 70 per cent of the total manganese ferroalloys in 2010, rising from 49 per cent in 2001. In 2010 this region was the major producer of HCFeMn (71 per cent of the world total), refined FeMn (69 per cent) and SiMn (70 per cent). In 2010 the region's total manganese ferroalloys production was 758 kt more than its consumption.
Although South Africa's production of manganese ferroalloys rose from 639 kt to 755 kt between 2001 and 2010, its share in world-wide production decreased from 8 per cent to 5 per cent. The increase in production is attributed to the increase in production of refined ferromanganese and silicomanganese during this period. Africa is a very small consumer of manganese ferroalloys, resulting in most of the production being exported to earn valuable foreign currency.
The major challenges preventing South Africa from being a significant supplier of manganese ferroalloys to industry worldwide are the rising cost of electricity and productivity of labour. These challenges could be addressed by industry to a limited extent, but involvement of other interested and affected parties is required to achieve a satisfactory solution.
Acknowledgements
Hilgard Rademeyer
Rodney Jones
Shana Baumgartner
Desh Chetty.
References
Anonymous. 2009a. Purity boosts manganese mining operations. http://www.informante.web.na/ [ Links ]
Anonymous. 2009b. Purity manganese aims for Namibia beneficiation project. http://minerals-and-metals.blogspot.com/2009/04/purity-manganese-aims-for-namibia.html [ Links ]
Assmang Limited. Machadodorp works. http://www.assmang.co.za/chrome_Machadodorp.asp [ Links ]
Basson, J., Curr, T.R., and Gericke, W.A. 2007. South Africa's ferro alloys industry - present status and future outlook. Infacon XI. Proceedings of the 11th International Ferroalloys Congress, New Delhi, India, 18-21 February 2007. pp. 3-24. [ Links ]
Brun, H. 1982. Development of refractory linings for electric reduction furnaces producing Mn alloys at Elkem A/S-PEA plant, Porsgrunn, Norway. Journal of the Institute of Refractories Engineers, Spring. p. 12. [ Links ]
Cairncross, B., Beukes, N., and Gutzmer, J. 1997. The Manganese Adventure -the South African Manganese Fields. Associated Ore & Metal Corporation. 236 pp. [ Links ]
Chetty, D. 2010. A geometallurgical evaluation of the ores of the northern Kalahari manganese deposit, South Africa. DPhil thesis, university of Johannesburg. [ Links ]
Christe, K. and Schneider, S. Chlorine. Encyclopaedia Britannica Online Academic Edition (2013). http://0-www.britannica.com.innopac.up.ac.za/EBchecked/topic/113561/chlorine [ Links ]
CLEGG, B. 2013. Chemistry in its element - potassium permanganate. http://www.rsc.org/chemistryworld/podcast/CIIEcompounds/transcripts/potassium_permanganate.asp [ Links ]
CREAMER, M. 2012. BHP Billiton shuts silicomanganese plant, impairment tests aluminium. Mining Weekly. 22 August 2012. [ Links ]
CREAMER, M. 2013. New R1bn manganese furnace signals beneficiation support - BHP Billiton. Engineering News. 6 March 2013 [ Links ]
Downing, J.H. 2012. Manganese processing. Encyclopaedia Britannica Online Academic Edition http://www.britannica.com/EBchecked/topic/361933/manganese-processing [ Links ]
Encyclopadia Britannica Online Academic Edition. 2012a. Manganese (Mn). http://www.britannica.com/EBchecked/topic/361875/manganese [ Links ]
Encyclopædia Britannica Online Academic Edition. 2012b. Pottery. http://www.britannica.com/EBchecked/topic/472867/pottery [ Links ]
Habashi, F. 1997. Handbook of Extractive Metallurgy, vol. 1. Wiley-VCH. [ Links ]
Hearn, A.M., Dzermejko, A.J., and Lamont, P.H. 1998. 'Freeze' lining concepts for improving submerged arc furnace lining life and performance. INFACON XIII. 8th International Ferroalloys Congress, Beijing, China; 7-10 June 1998. pp. 401-426. [ Links ]
International Manganese Institute. 2010. IMnI 2010 Mn Public Report 2010. www.manganese.org [ Links ]
Jones, R.T. 2010. Pyrometallurgy in Southern Africa. http://www.pyromet-allurgy.co.za/PyroSA/index.htm [ Links ]
Kalahari Resources. Kalagadi manganese. http://www.kalahariresources.co.za [ Links ]
Kazadi, D., Groot, D.R., Pöllmann, Η., De Villiers, J.P.R., and Redtmann, T. 2013. utilization of ferromanganese slags for manganese extraction and as cement additive. International Conference on Advances in Cement and Concrete Technology in Africa, Johannesburg, 28-30 January 2013. http://www.accta2013.com [ Links ]
Olsen, S.E., Tangstad, M., and Lindstad, T. 2007. Production of Manganese Ferroalloys. Tapir Academic Press, Trondheim, Norway. p. 247. [ Links ]
Olsen, S.E. and Tangstad, M. 2004. Silicomanganese production - process understanding. INFACON X: Transformation Through Technology, Cape Town, South Africa, 1-4 February 2004. pp. 231-238. [ Links ]
Parker, J.A.L. and Loveday, G.K. 1996. Recovery of metal from slag in the ferro-alloy industry. Hidden Wealth: Proceedings of a Conference. South African Institute of Mining and Metallurgy, Johannesburg. pp. 7-15. [ Links ]
Reisfeld, R. 2001. Optical properties of rare earth and transition element doped glasses. Encyclopedia of Materials: Science and Technology. Elsevier. pp. 6472-6477. [ Links ]
Reti, R. 2001. Silver: alloying, properties and applications. Encyclopedia of Materials: Science and Technology. Elsevier. p. 8621. [ Links ]
Reuter, M.A., Xiao, Y., and Boin, U. 2004. Recycling and environmental issues of metallurgical slags and salt fluxes. Vii international Conference on Molten Slags Fluxes and Salts. Symposium Series S36. South African Institute of Mining and Metallurgy, Johannesburg. pp. 349-356. [ Links ]
South African Academy of Engineering (SAAE). The Hendrik van der Bijl Memorial Lectures. http://www.saae.co.za/vdbijl [ Links ]
Transalloys. http://www.transalloys.co.za [ Links ]
Van Averbeke, N. 2005. SAMI - South Africa's Minerals Industry 2004-2005. Department of Mineral Resources, Pretoria. p. 195. [ Links ]
Van Reenen, J.H., Thiele, Η., and Bergman, C. 2004. Recovery of chrome and manganese alloy fines from slag. INFACON X Transformation Through Technology, Cape Town, South Africa, 1-4 February 2004. pp. 548-554. [ Links ]
Wadhavkar, A. 2012. Match Corporation. Personal Communication. [ Links ]
World Steel Association. 2009. Annual crude steel production 2000 - 2009. http://www.worldsteel.org [ Links ]
World Steel Association. 2010. Annual crude steel production 2010. http://www.worldsteel.org [ Links ]
Zamanco Minerals. 2012. Serenje manganese project. http://www.zamancominerals.com/media/20589/120911_zam_options-analysis.pdf [ Links ]
Paper received Apr. 2013
Revised paper received May 2013
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN 2225-6253.
















