Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 n.8 Johannesburg Jan. 2013
CONFERENCE
Fluorinated melt-extruded thermoplastic linings in off-gas pipelines of sulphuric acid production plants and in tailor-made equipment for sulphur oxide and sulphuric acid handling
M. Lotz
Quadrant EPP AG, Lenzburg, Switzerland
ABSTRACT
Gases containing sulphuric acid and sulphur oxide (SO2 , SO3 ) can lead to severe corrosion problems, in particular in high concentrations combined with water vapour. If acid condensation takes place, this can lead to the extremely destructive dew-point corrosion. In these cases, even materials like titanium and stainless steels can be attacked in the aggressive environments, or suffer variable but significant degrees of material loss by surface degradation.
An excellent option for solving the corrosion problems induced by sulphuric acid and sulphur oxide is linings made from fluorinated melt-extruded thermoplastics. Those can be divided into partially fluorinated polymers like polyvinylidenefluoride (PVDF) and ethylene-chlorotrifluoroethylene (ECTFE), and fully fluorinated materials like tetrafluoroethylene-hexafluoropropylene (FEP) and tetrafluoroethylene-perfluoroalkylvinylether (PFA). The latter have a much higher temperature and chemical resistance than partially fluorinated thermoplastics, but share their outstanding processing properties. Fully fluorinated melt-extruded thermoplastics extend the application range of the lining solutions remarkably. The linings can be installed in sheets, tanks and reactors for liquid sulphuric acid, as well as in scrubbers and pipelines for the conversion and transport of sulphur oxide and sulphuric acid gases and fumes, including the shut-off valves for flow regulation.
An introduction to and comparison of the lining materials and installation methods will be presented, illustrated by means of selected relevant applications. Application ranges for partially and fully fluorinated melt-extruded thermoplastics will be discussed against the background of the chemical compounds and temperatures involved. Case studies of two recently installed and successfully running pipelines in sulphuric acid producing metallurgical plants will be shown in detail. One is a fibre-reinforced plastic (FRP) reinforced FEP liner pipe for off-gases containing droplets of sulphuric acid. The other is FEP-lined blast spools made from stainless steel, also installed in gas streams containing sulphuric acid. Fabrication methods for transition forms, over-dimensional pipes, and other tailor-made parts will be explained, and relevant quality standards and best practices will be discussed.
Keywords: extrusion, fabric backing, ECTFE, PFA, PTFE, PVDF, FEP, fluoropolymer, fluoroplastic, fully fluorinated, partially fluorinated, dual laminate, FRP, bonded steel lining, liner pipe, loose lining, fix point lining, sulphuric acid, sulphur oxide, corrosion, melt-extrusion.
Introduction
Sulphur oxide and sulphuric acid production
Many metals, including cadmium, cobalt, copper, lead, molybdenum, nickel, silver, and zinc occur in nature as sulphidic ores. Smelting, leaching, and electrowinning processes are applied to separate metals and sulphur compounds. These processes generate sulphur oxides (SO2 and SO3) and sulphuric acid, or require sulphuric acid to solubilize the ore minerals (Beale, 1985; Woods, 2010). Since the mid-1970s, sulphuric acid production plants connected to smelters have played a pivotal role in environmental pollution control (Plasket and Ireland, 1976). Other industrial sources for sulphur oxides and sulphuric acid, respectively, are the Claus process, spent sulphuric acid regeneration by thermal decomposition, as well as treatment of off-gases from fossil- and waste-fuelled power production. SO2-bearing gases thereby often serve as raw material for sulphuric acid production via the catalytic conversion of SO2 to SO3 (Davenport et al., 2006).
Steel materials
Particular requirements exist for the material of construction used to build the process equipment. SO3 and sulphuric acid behave as extremely corrosive substances, and mild steel can be corroded even by SO2 (Sun and Nesic, 2007) unless an appropriate corrosion protection is applied. In addition, sulphuric acid has a dual chemical nature, acting as a reducing acid at concentrations up to 70%, but as an oxidizing acid at higher concentrations. Steel materials of construction (MOCs) including high-nickel alloys, behave differently during exposure to sulphuric acid, depending on the concentration and temperature. However, all steels undergo a certain material loss over time, which has to be taken into account for the applications. Furthermore, exotic alloys are usually comparatively expensive material solutions (Sulphuric Acid on the Web™, 2005).
Plastic linings
Where applicable from the temperature requirements and mechanical loads, plastics and plastic liners are an alternative to metals. Plastic liners can be used either on carbon steel or, less frequently, stainless steel structures. Lined fibre-reinforced plastic (FRP) composites are another option. Plastics also offer an excellent material alternative where otherwise expensive MOCs with partially limited availability, such as nickel alloys, titanium, or tantalum would be required (Lyons, 2007; Sulphuric Acid on the Web™, 2005).
Fluoroplastics
Advantages compared to other plastic polymers
Plastics like polypropylenes, polyvinylchlorides, and polyeth-ylenes are already used in large volumes in the chemical industry. However, they can be chemically and thermally unstable in very aggressive conditions, at high temperatures, or in the presence of a variety of media. In fluoroplastics, the carbon backbones of the molecules are 'shielded' by fluorine atoms against chemical attack. Both the carbon-carbon (bond energy 607 kJ/mol) and carbon-fluorine bonds (bond energy 552 kJ/mol) are chemically very strong, equipping the fluoroplastics with extraordinary resistance against chemicals and heat. This also makes fluoroplastics resistant in the most aggressive sulphur oxide and sulphuric acid environments. Further benefits, for example, are non-stick properties, a low wettability by aqueous media, and low friction, which can be beneficial for numerous applications. Unlike metals, fluoro-plastics do not undergo material loss by oxidation processes on the surface exposed to media (Ebnesajjad and Khaladkar, 2004).
Classification
Fluoroplastics can be divided into plastics processed by sintering on the one hand and melt-processible plastics on the other. With respect to lining materials, melt-processing usually means extrusion processes, from which lining foils and pipes are created.
Another fundamental classification can be made on basis of the degree of fluorination, which is one of the major determinants of the chemical resistance of the plastics (Ebnesajjad and Khaladkar 2004). Whereas fully fluorinated plastics are occupied solely by fluorine atoms, partially fluorinated plastics possess fluorine, hydrogen, and chlorine atoms (the latter only in ECTFE) in their side chains (Table I).
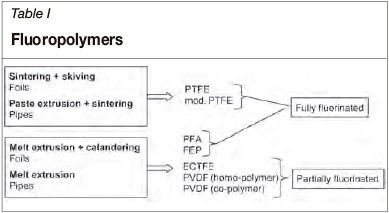
Sintered fluoroplastics usually refers to polytetrafluo-roethylene (PTFE) and its modifications. Examples of melt-extruded fluoroplastics are PVDF, ECTFE (partially fluorinated), as well as FEP and PFA (fully fluorinated).
For the manufacturing of linings for the protection of chemical process equipment, melt-extruded fluoroplastics have a number of technical advantages. In contrast to sintered fluoroplastics, extruded fluoroplastics form a liquid melt, leading to outstanding welding and thermoforming properties, which renders possible the fabrication of tailor-made components with high process safety. This is a vital requirement for handling of the often extremely aggressive, hot, and sometimes even toxic chemical media the materials are used in. Furthermore, the liquid melt of melt-processible fluoroplastics allows the direct embedding of fabric backings into the lining materials, which is crucial for obtaining high liner bond strengths in bonded lining applications, and micro-pores (typical for sintered materials) are not present in melt-extruded fluoroplastics.
For these reasons, this report is focussed on the application of melt-extruded fluoroplastics.
Application ranges in sulphur oxide and sulphuric acid
In case of partially fluorinated fluoroplastics, the media type and concentration have a significant impact on the chemical resistance (Table II).
Whereas a clear decrease in the maximum service temperature can be observed for PVDF at sulphuric acid concentrations higher than 70%, the data for ECTFE shows no significant impact of high sulphuric acid concentrations. In general, ECTFE has a better chemical resistance than PVDF in most chemical media (apart from a few exceptions), which is also reflected by the sulphuric acid resistance data.
The usage of partially fluorinated polymers apparently cannot be recommended if higher concentrations of SO3 are present in the process. This includes process streams containing oleum (sulphuric acid oversaturated with SO3).
In contrast, no limitations in chemical resistance can be observed for fully fluorinated polymers (Table II).
In terms of chemical resistance, fully fluorinated polymers can be used in sulphur oxide and sulphuric acid environments up to their fundamental temperature application ranges of 205°C (FEP) and 260°C (PFA). Also, application with significant amounts of SO3 and oleum is possible.
Lining technology
Requirements
A major requirement for the lined system is posed by the temperature profile of the application. Up to approximately 120°C, no principal restrictions exist on the choice of melt-extruded fluoroplastic-lined system (given that the fluoro-plastic polymer and material is chosen properly and also depending on the equipment manufacturing quality).
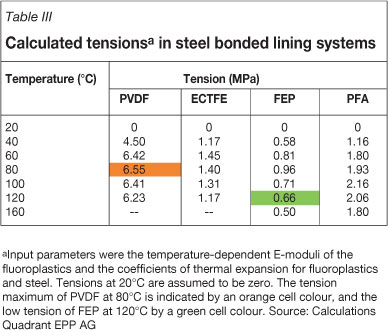
At temperatures above 120°C, a bonded steel lining cannot be recommended. One reason is the achievable bond strength between the liner and steel, which is usually less than the bond strengths achievable in FRP. Further reasons are the relatively large differences in the thermal expansion of the thermoplastic liner and the steel backing (the ratio of the thermal expansion of thermoplastic to steel is approximately 8:1), leading to tensions in the lined system. It is noteworthy that the temperature at which the maximum tension is reached depends on each individual polymer, since softening of the thermoplastic with increasing temperature leads at some point to a decrease in the tension induced by expansion (Table III). For example, PVDF in a bonded lining has a tension maximum at about 80°C, and frequent temperature cycles through 80°C cannot be recommended. However, static temperatures of 80°C in a bonded PVDF lining are usually not problematic.
FRP dual laminates are FRP structures such as vessels, columns, reactors, and pipes lined with thermoplastics including fluoroplastics. Application temperatures between 160°C and 180°C (PFA-FRP dual laminate) are reported. Whether these high temperatures can be reached of course depends also on the geometry, the quality of the lining materials, and equipment fabrication, as well as on whether a thermal insulation is applied from the outside (which influences the temperatures in the composite layers).
At temperatures above 180°C, however, no bonded lining systems can be recommended, since the resin-based bonded connection does not provide sufficient bond strength. The alternative is mechanically attached liner foils, usually PFA, applicable up to temperatures of 260°C (maximum application temperature of PFA).
Mechanical fixing is either by fix points (fix point lining) or by clamping (clamped or loose lining) into flanges of chutes, nozzles, and other components. The fix points basically consist of bolts, washers, and hexagonal nuts holding the foil, protected against chemical attack by welded PFA caps. Mechanically fixed linings are found mainly in condensing heat exchangers and housings of fossil- or waste-fired power plants, but also in condensing heat exchangers used for sulphuric acid production processes (Figure 1).

Other common requirements for the selection of the lined system are, for example, high positive (bonded steel linings preferable) or high negative (FRP dual laminates preferable) pressures, permeation-prone media like chlorine, hydrochloric (HCl), and hydrofluoric (HF) acid (FRP dual laminates preferable), protection against corrosive attack from the outside (FRP dual laminates preferable) or other specifications arising from the application. For example, containers for transporting chemical media are normally made from steel, and hence a bonded steel lining is chosen.
Reliable manufacturers of fluoroplastic lining materials also possess comprehensive technical data about applications, and can recommend a choice of lining method for particular process conditions.
Bonded steel lining
A good bonded steel lining starts with a well prepared metal structure. Ideally, the metal structure is already built with a design tailored to thermoplastic lining. Sharp edges, complex shapes, and other features that make the lining complicated and expensive should be avoided to the maximum possible extent. A well prepared and clean inner steel surface is crucial in order to achieve the desired high bond strengths. A steel surface cleanliness according to Swedish standard SA 2 1/2 is state-of-the-art and should be achieved immediately before the liner is glued onto the steel; the formation of rust or contamination of the steel surface between metal surface preparation and liner application should be avoided by all means. Primers can be used, according to the individual lining protocol of the equipment fabricator. For the lining, knitted fabric-backed lining materials (lining laminates) are used (Figure 2).

The lining materials are tailored, thermoformed, and subsequently glued into the steel structure with an excess of resin (usually epoxy resin) in order avoid air bubbles behind the liner (Figure 3).

After the curing process, the liners are welded from the media-exposed side in order to seal the surface.
FRP dual laminates and FRP-reinforced liner pipes
The fabrication of FRP dual laminates starts with creation of the liner. A 'liner body' is created by thermoforming, tailoring, and welding from knitted fabric-backed lining laminates. Concave forms like tank bottoms can also be created by thermoforming of lining laminates connected by straight welds. This saves fabrication time and in addition contributes to the equipment quality, since the number of weld seams is reduced to a minimum. Furthermore, the straight welds can be done by machine (butt mirror or flow fusion) welding if the fabricator has such equipment available. This can further improve the weld seam and in consequence the equipment quality.
For the FRP reinforcement, tanks with rotational symmetry and pipes (before connection of the straight pipe segments by welding) can be reinforced in partially automated filament winding machines (Figure 4).

For other shapes or if a filament winding device is not available, hand lamination is applied.
As the quality standard for the fabrication of FRP dual laminates, including basic requirements for the semi-finished products, an excellent standard is in place (American Society of Mechanical Engineers, 2011), and hence this topic will not be addressed here further.
A particular advantage of FRP-reinforced liner pipes is that the pipe sections can be connected flangeless by welding. PTFE-lined steel pipes, for example, usually have flanges at least every 6 m or even 8 flanges in compensation loops. Flanges are often subject to leakage problems and can increase the maintenance cost significantly, but these problems can mostly be avoided by using FRP-reinforced liner pipes. Of course, the latter can also be equipped with flanges and instrumentation where required for the chemical process.
Fix point or loose linings
In contrast to bonded steel linings and FRP dual laminates, non-fabric-backed lining materials, so-called natural foils and pipes, are used for mechanically fixed linings; since no resins or adhesives are used, there is no need for fabric backings or surface modifications.
Partially fluorinated plastics like PVDF and ECTFE, due to their mechanical properties and comparatively stiff nature, do not fulfil the mechanical requirements for a fix point or loose lining. Hence, PVDF and ECTFE can be practically ruled out for a mechanical fixation, at least for the application of corrosion protection with aggressive liquid or gaseous media.
PFA is used almost exclusively, and only in rare cases is FEP used. This on the one hand has to do with the applications themselves, which usually require the high-temperature stability of PFA. In addition PFA has an outstanding resistance against repeating mechanical movements in the foil (flex life), which is another advantage for a mechanical fixed lining, compared to FEP. Regular foil thicknesses are 1.5 mm or 2.3 mm, but in special cases can be higher (e.g. 2.8 mm or 3.8 mm).
PFA (likewise all fluoroplastics) is a semi-crystalline polymer. Hence, it can shrink to some extend after heating due to further crystallization events in the foil. The shrinkage in the foil can be of relevance for particular equipment components exposed to high temperatures. It is recommended that the PFA foil supplier is asked for the information, if shrinkage is expected to be critical. As the effect is most pronounced close to the melting temperature (290-310°C for PFA), the shrinkage should also be measured as close as possible to the melting temperature (Quadrant Symalit recommendation: measurement at 250°C).
In case of a fix point lining, the number of bolts should be chosen in accordance with the mechanical loads expected. This can vary between very few fix points in regions of low loads and sections 'peppered' with fix points. The latter, for example, can be hoods with high flow velocities or certain negative pressures.
The weld seams can be unburdened from movement and tension in the lining by an appropriate positioning of the fix points relative to the weld seams. For sealing of the fix points, either injection-moulded or thermo-formed PFA caps can be used, which has to be taken into account during the weld process. Straight welds in the foils can be done either as V- or X-shaped weld seams (X-shaped rather for thicker foils), or as overlapped weld seams.
Examples
FRP dual laminate storage tanks and vessels for sulphuric acid
A frequent application for FRP dual laminates is storage vessels for sulphuric acid. A case history (Quadrant EPP AG, 1993a) for example, describes cylindrical tanks 8 m x 8.5 m made from FRP with 3 mm PVDF and 2.3 mm ECTFE lining. The tanks were placed outside at air temperatures between -30°C and +30°C without thermal insulation. The sulphuric acid had a temperature of 70°C and varied in concentration between 80% and 85%.
As a second example, 2.3 mm PFA-FRP dual laminate storage tanks are reported (Quadrant EPP AG, 2002) holding 96% sulphuric and 37% hydrochloric acid at 49°C.The contained acids were of high-purity grade, and contact with the metal could be avoided using the PFA lining.
FRP dual laminate evaporators for spent sulphuric acid
Evaporators with dimensions of 2.8 m x 3.5 m and associated piping were built as 2.3 mm FEP-FRP dual laminate (Quadrant EPP AG, 1993b). The system operated at 115°C, circulating 70% sulphuric acid under full vacuum. In addition, 300 g/l iron sulphate crystals were suspended as solids. For this application, the FEP lining had an additional benefit due to its good abrasion resistance (Table IV).

FRP dual laminate oversized T-fitting for sulphuric acid
A T-fitting with five flanges and dimensions of 1.4 m x 3.5 m had been built as 2.3 mm FEP-FRP dual laminate (Figure 5).

The medium transported was 98% sulphuric acid at a temperature of 120°C and a pressure of 1 bar. The T-fitting, due to its dimensions, reflects elements of both a tank and pipeline construction. For example, it was not built from liner pipes, but was created by tailoring, thermo-forming, and welding of FEP knitted fabric-backed lining laminates.
Shut-off valves made from bonded lined steel with FRP housings for sulphur oxide- and sulphuric acid-containing flue gas
The flue gas from the metal production plants in the reported case (Quadrant EPP AG, 2004) was loaded with calciner (TiO2), 4.4 g/m3 SO3, and 30 g/m3 sulphuric acid. The absolute operating pressure was between -96 kPa and +104 kPa. Further requirements for the lining solution were suitability for temperatures of up to 90°C and high abrasion resistance in the presence of the solid particles contained in the gas stream.
In order to meet these requirements, steel flaps of shut-off valves with diameters DN 1000, DN 2500, and DN 2800 were protected with a bonded lining of 2.3 mm ECTFE, using a special epoxy resin as adhesive to meet the high temperature requirements. The flaps were held in round frames, manufactured from vinyl ester FRP. The solution lasted at least 12 years without any interruption, according to the case history data. However, the actually runtime achieved might be much higher and the valves might still be in service.
FRP dual laminate pipe line for sulphuric acid-containing off-gas
For a sulphuric acid producing process associated with metallurgy, a 2.3 mm FEP-FRP dual laminate pipeline was installed in order to replace an older metal pipeline (Quadrant EPP AG, 2011a), which suffered from corrosion. The medium transported was mainly dry nitrogen gas containing droplets of 97% sulphuric acid at a temperature of 80-85°C and flow rate of about 35 000 m3/h at a pressure of -0.2 bar (vacuum).
The pipe had an outer diameter of 1000 mm and a length of approximately 180 m. The FEP liner, chosen because of the practical inertness of FEP against sulphuric acid, was reinforced with vinyl ester FRP, which also provided a good chemical resistance on the outside (Figure 6).

In order to produce this oversized diameter pipeline, knitted glass fabric-backed FEP lining laminate sheets were first produced in lengths corresponding to the outline of the pipe. The laminate sheets were welded each with a single longitudinal seam to yield the inner liner pipe. The individual liner pipe sections then were connected by welding.
Steel blast spools with bonded lining for sulphuric acid-containing gas
For a metallurgical application, blast spools were fabricated for the transport of gases with a high sulphuric acid content at temperatures of up to 120°C. Overall, the conditions were outstandingly aggressive and even materials like titanium did not provide a satisfying durability in the application. A first loose lining of the affected titanium parts (not illustrated) solved the corrosion problems and demonstrated the suitability of FEP as a MOC.
The solution for the blast spools accordingly was an AISI 316 (V4A, stainless steel) structure with a bonded 2.3 mm FEP lining (Figure 7; Quadrant EPP AG, 2011b). A further benefit of FEP for the application was the particularly low tension values in a bonded steel lining at 120°C (Table III), potentially also contributing to long runtimes of the lined equipment.

Stainless steel was chosen in order to provide sufficient protection against corrosion on the exterior. Vacuum thermo-forming and tailoring of the knitted fabric-backed lining laminates was applied in order to fit the lining into the blast spool geometries, and hot gas welding was used to seal the inner liner surface. As adhesive, a dedicated epoxy resin and hardener system was used to withstand the high temperatures.
Conclusions
Linings fabricated from melt-extruded fluoroplastics are valuable MOCs for the production and handling of sulphuric acid and sulphur oxides. In many applications, fluroroplastic linings outperform even expensive or difficult-to-obtain available metals. The choice between partially and fully fluorinated polymers allows the use of melt-extruded fluoro-plastic linings for all concentrations of sulphuric acid, even up to high temperatures. Diverse lining technologies, including established best practices, are applicable in order to find the best lining solution for different process requirements. Tailor-made individual solutions are possible.
Acknowledgement
The author would like to thank Panergetic AG, Angenstein AG, PRP Plastic OY, Plasticon Germany GmbH, and Plastilon OY for providing the images of the lined equipment.
References
American Society of Mechanical Engineers. 2011. Reinforced thermoplastic corrosion resistant equipment (ASME RTP-1-2011). [ Links ]
Beale, C.O. 1985. Copper in South Africa - Part 1. Journal of the South African Institute of Mining and Metallurgy, vol. 85, no. 3. pp. 73-80. [ Links ]
Davenport, W.G., King, M.J., Rogers, B., and Weissenberger, A. 2006. Sulphuric acid manufacture. Southern African Pyrometallurgy, Cradle of Humankind, South Africa, 5-8 March 2006. [ Links ]
Ebnesajjad, S. and Khaladkar, P. 2004. Fluoropolymers. Applications in Chemical Processing Industries. William Andrew, New York. [ Links ]
Francis, R. 2009. The performance of stainless steels in concentrated sulphuric acid. Stainless Steel World, vol. 22. [ Links ]
Lyons, E. 2007. Case history of duallaminate life cycle cost analysis vs. metallic piping. Corrosion 2007, Nashville, Tennessee, 11-15 March 2007. NACE. [ Links ]
Plasket, R.P. and Ireland, D.A. 1976. Ancillary smelter operations and sulphuric acid manufacture at Impala Platinum Limited. Journal of the South African Institute of Mining and Metallurgy, vol. 77, no. 1. [ Links ]
Sulphuric Acid on the Web™. 2005. Materials of construction - acid resistant linings. http://www.sulphuric-acid.com/techmanual/Materials/materials_linings.htm [Accessed 11 Jul. 2013]. [ Links ]
Sun, W. and Nesic, S. 2007. A mechanistic model of H2S corrosion of mild steel. Corrosion/2007. NACE, Houston, TX. [ Links ]
Quadrant EPP AG. 2011. Chemical resistance data base. Symalit Fluoroplastics Materials. http://www.quadrantplastics.com/en/support/chemical-resistance-information/symalit-R-fluoroplastics-materials.html [Accessed 9 Jan. 2013]. [ Links ]
Quadrant EPP AG. 2002. Symalit case history: storage tanks for high purity acids. http://www.quadrantplastics.com/contact/contact_list/166.html [Available on request]. [ Links ]
Quadrant EPP AG. 1993a. Symalit case history: storage vessels. http://www.quadrantplastics.com/contact/contact_list/166.html [Available on request]. [ Links ]
Quadrant EPP AG. 1993b. Symalit case history: acid recovery evaporators. http://www.quadrantplastics.com/contact/contact_list/166.html [Available on request]. [ Links ]
Quadrant EPP AG. 2004. Symalit case history: shut-off valve. http://www.quadrantplastics.com/contact/contact_list/166.html. Available on request. [ Links ]
Quadrant EPP AG. 2011a. Symalit case history 11-1: mining and sulphuric acid production. http://www.quadrantplastics.com/contact/contact_list/166.html [Available on request]. [ Links ]
Quadrant EPP AG. 2011b. Symalit case history 11-2: mining and sulphuric acid production. http://www.quadrantplastics.com/contact/contact_list/166.html [Available on request]. [ Links ]
Woods, R. 2010. Extracting metals from sulfide ores. Electrochemistry Encyclopedia. http://electrochem.cwru.edu/encycl/ [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN 2225-6253.
This paper was first presented at the, 4th Sulphur & Sulphuric Acid 2013 Conference, 3-4 April 2013, Sun City, Pilanesberg, South Africa.














