Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 no.6 Johannesburg Jun. 2013
Kinetic studies on the leaching reactions in the autoclave circuit of the Tati Hydrometallurgical Demonstration Plant
B.D. PaphaneI; B.B.M. NkoaneII; O.A. OyetunjiII
IBasic Science Department, Botswana College of Agriculture, Gaborone, Botswana
IIDepartment of Chemistry, University of Botswana, Gaborone, Botswana
SYNOPSIS
Since the commissioning of the Tati Hydrometallurgical Demonstration Plant (HDP), which produces Ni and Cu cathodes as well as Co salt from sulphide concentrate employing Activox® technology for leaching, no kinetic data has been generated from the plant under normal operating conditions, from start-up to steady state. Therefore, this study seeks to determine the rate of reactions in the leaching circuit with a view to obtaining kinetic data for the system under the current operating conditions. Within the five-compartment autoclave operating at temperatures of 105°C to 110°C and 1100 kPa pressure, the extraction of Ni, Cu, and Co ions into solution is achieved through the oxidation of the sulphide concentrate using approximately 50 kg H2SO4 per ton, 10 kg NaCl per ton, and 1.21 kg O2 per kilogram of S2-. The autoclave reactions showed complex kinetics, with the reactions proceeding via some intermediate(s) before the formation of products. The results suggest that the reactions were dependent on the size of the metal ion of interest and the concentrations of the metal ions in the Tati concentrate as well as in chloride leaching. The order of the leaching rate constants was Fe2+ > Cu2+ > Ni2+ > Co2+ in all the compartments studied. The rates of leaching were also found to decrease as the concentrate moves from compartment 1 to compartment 4. For compartments 1 to 3, leaching was observed to be preceded by dilution of the repulp liquor (copper raffinate). The leaching rate for compartment 5 was slightly lower than that of compartment 1 but higher than the rates of compartments 2, 3, and 4 for all the metals studied.
Keywords: kinetics, hydrometallurgical plant, Activox® leaching, autoclave circuit, absorption, rate constants.
Introduction
Until 2009, Tati Nickel Mining Company (TNMC) operated a hydrometallurgical demonstration plant (HDP) in Botswana using the Activox® leaching technology developed by LionOre Technology (now Norilsk Process Technology, NPT) to produce Ni and Cu cathodes and Co salt (mostly carbonate) (Western Minerals Technology, 2004). The Activox® leaching process combines fine milling, using a suitable power-efficient stirred mill, with low-temperature pressure oxidation. The mild operating conditions of temperature (about 105°C) and pressure (about 1 100 kPa) simplify the engineering requirements and reduce costs while maintaining the advantages of pressure oxidation compared to conventional pressure oxidative leaching (POX), which operates at temperatures over 200°C and pressures of over 2 200 kPa (Angove et al., 1993; Corrans et al., 1995; Johnson et al., 1993).
The main purpose of the leach circuit is to selectively dissolve (leach) nickel, copper, and cobalt from the finely milled flotation concentrate. The resultant metal-rich liquor and oxidized solids are advanced to the rest of the Tati HDP, which employs the use of known downstream technologies such as partial neutralization (PN), countercurrent decantation (CCD), solvent extraction (SX), and electrowinning (EW) for Cu and Ni recovery (Western Minerals Technology, 2004; Nel and van den Berg, 2009 ).
The autoclave circuit receives feed slurry from the concentrate preparation and milling circuits. The received slurry should have a feed size of P80 of around 10 µm after undergoing fine grinding in the milling circuit. This creates more surface area for oxidative attack in the autoclave, hence reducing the required retention time by increasing the rate of autoclave chemical reactions (Western Minerals Technology, 2004).
The extraction of Ni, Cu, and Co in solution is achieved through the oxidation of the sulphide within a five-compartment autoclave operating at temperatures of 105°C to 110 °C and 1100 kPa pressure, by the addition of approximately 50 kg H2SO4 per ton, 10 kg NaCl per ton, and 1.21 kg O2 per kilogram S2-. The primary leaching of the base metals in the autoclave circuit can be represented as follows:
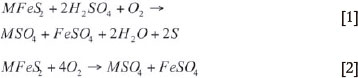
where M = Ni, Co, Cu
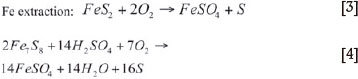
Reactions [1] to [4] are exothermic, hence heat control is very important in order to keep the temperature between 105°C and 110°C. This is done by using vacuum flash cooling (compartments 1 and 2), as well as direct-quench water cooling (all compartments) (see Figure 1).
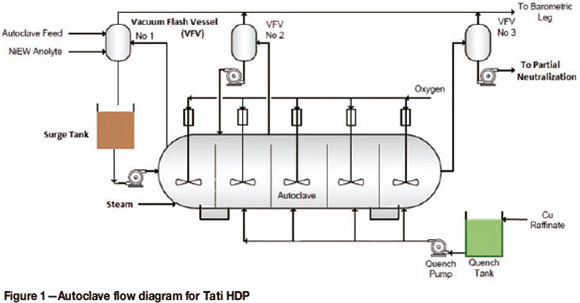
Figure 1 shows a schematic flow diagram for the leach circuit, which is further described below. The rest of the flow sheet is described elsewhere (see Western Minerals Technology, 2004; van der Berg and Nel, 2009).
The mill discharge is pumped to autoclave vacuum flash vessel (VFV) no. 1 from the autoclave feed tank, via the autoclave feed pump. Ni electrowining (Ni EW) anolyte is also pumped directly into autoclave flash vessel no. 1 from the spent electrolyte storage tank via the spent electrolyte pump. The slurry is agitated in each compartment. Quench and steam are injected directly underneath the agitators for effective mixing (steam is required only for the initial heating phase on start-up). The slurry overflows the baffles that separate each compartment, gradually moving through the autoclave until it is discharged from compartment 5. Compartment 1 is fed from VFV no. 1 via the flash vessel no. 1 pump. Slurry is removed for temperature control via autoclave compartment 1 flash valve to autoclave VFV no. 1 into the surge tank as a recirculating load. The slurry is then re-injected into compartment 1 via a surge tank pump. In compartment 2, slurry is removed for temperature control via autoclave compartment 2 flash valve to autoclave flash vessel no. 2 as a recirculating load. The slurry is then re-injected into compartment 2 via the flash vessel no. 2 pump. Slurry is discharged from compartment 5 via compartment 5 discharge flash valve to the discharge flash vessel (flash vessel 3), from which it leaves the circuit through the autoclave discharge flash vessel pump and discharges to the partial neutralization surge tank. Steam is removed from autoclave flash vessels 1, 2, and 3 via a common vacuum manifold due to the negative pressure generated by the liquid ring vacuum pump to the flash vessel barometric leg. The liquid ring vacuum pump requires liquid (water) which is supplied to its reservoir by either potable water (normally) or the cooling water circulation pump, which is selected by opening or closing manual isolation valves. Each of the five autoclave compartments has a sampling bomb equipped with a valve. (Norilsk Process Technology, 2007).
Since the Tati HDP was commissioned in 2004, no leaching kinetic studies have been investigated under normal operating conditions, from cold start-up to steady state. Welsh and others (Welsh and Barclay, 2006; Welsh, 2006), have written different technical notes on kinetic modelling of particles during Activox leaching using the shrinking core and shrinking sphere model. However, the data obtained in these reports was not sufficient to provide detailed kinetic parameters so as to propose the mechanism for the leaching reactions.
Kinetic studies allow the prediction of how quickly a reaction mixture approaches equilibrium, although a thermo-dynamically stable complex may not necessarily be kinetically stable. Moreover, kinetic studies lead to an understanding of the mechanisms of the chemical reactions (Jones and Atkins, 2000). Depending on the nature of the reaction and the rate at which the reaction proceeds, different techniques have been employed to follow kinetic studies. Most of these are classical techniques that are used to measure slow reactions in hours or minutes (Pilling and Seakins 2001). One of such classical techniques is the 'absorption technique', used in this study.
The current study focuses on kinetic investigations of the dissolution of nickel, copper, cobalt, and iron in the autoclave circuit of the Tati HDP. Such information will be useful in proposing a mechanism for the oxidative attack on these base metals. Therefore, the main objective of the current research was to determine and compare the leaching rate constants for the different metals of interest under the same operational autoclave leaching conditions.
Methods and materials Sampling
Assuming that the slurry from the leach circuit is relatively inert to oxidation from atmospheric oxygen and pressure, slurry samples were taken from six sampling sites in and around the autoclave; namely, the surge tank and each of the five compartments. The sample from the autoclave discharge line was assumed to be the same as that taken from compartment 5. The samples were taken at 5 minute intervals for the first one hour from the time that the leaching circuit was started. Subsequent samples were taken at 10 minute intervals for another four hours, by which time the leaching reaction was assumed to be completed and the process having reached steady state. Three samples were obtained at each sampling time (n=3). The samples were immediately stored in the refrigerator to minimize any possibility of self-oxidation. The slurry samples were then filtered under suction to separate solids from solutions. Both the solid and solution portions were kept for further analyses.
Equipment
Solution electronic spectra were recorded on a Shimadzu UV-2501PC UV-Vis spectrophotometer. Matrix-matched standards were prepared from analytically pure acidified 1000 ppm stock standard solutions of Ni2+, Cu2+, Fe2+, and Co2+ for the different metals of interest. The matrix-matched solutions were used for background correction arising from any matrix interferences. A solution of the autoclave (AC) discharge sampled at 400 minutes was scanned over the range 1100-200 nm. The matrix-matched standards were also scanned over the same wavelength region. The scanned spectra were independently superimposed onto the AC discharge spectra for each metal of interest to identify regions of maximum, but unblocked, absorbance for the metals. This allowed monitoring the kinetics of the reactions at a fixed wavelength for each metal as follows: Cu2+, λ = 279.00 nm; Ni2+, λ = 283.00 nm; Co2+, λ = 291.00 nm; and Fe2+, λ = 262.00 nm.
The kinetics were followed under pseudo-first-order conditions since under Activox autoclave conditions, sulphuric acid (99%) and oxygen (100%) are at much higher concentrations than those of the metals of interest (see Table I) (Western Minerals Technology, 2004). All kinetic studies were followed to completion. The pseudo-first-order rate constants (k) were obtained from linear regression plots of ln (A∞ - At) versus time where At and A∞ are the absorbances at time = t and time = ∞, respectively. The k values are averages of three runs for each analysis as previously determined (Oyetunji et al., 2006).
Results and discussion
As mentioned earlier, autoclave reactions are subjected to pseudo-first-order conditions since the oxidizing agents, H2SO4 and O2, are at concentrations that are more than ten times that of the sulphide metals. The extent of leaching within the six sampling points, as the slurry moves from the surge tank through the entire length of the five-compartment autoclave and the autoclave discharge line, was studied and the results are discussed in the following sections.
Autoclave discharge
Figure 2 shows a reaction curve for Ni obtained for autoclave discharge solution from the time the concentrate was added to the autoclave (cold start-up) to steady state. Similar reaction (absorbance) curves were obtained for Co, Cu, and Fe.
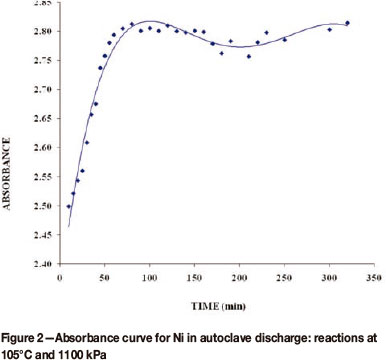
The plot of ln(A∞ - At)versus time for the Ni autoclave discharge reaction gave a straight line, showing that the reaction is first order with respect to the oxidizing acid, as illustrated in Figure 3. All the other plots for the discharge of Co, Cu, and Fe were similar to Figure 3.
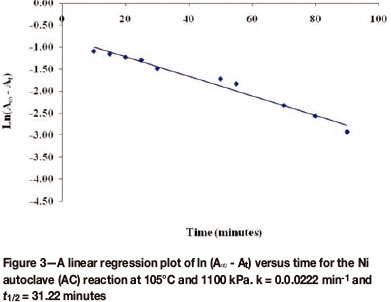
As explained earlier, k, is a composite rate constant whose composition can be determined only by using the method of initial rates on a laboratory scale, since at that level the concentrations of all reactants can be easily monitored. Inspection of the kinetic plots shows that autoclave reactions proceed via an intermediate before giving the product according to Equations [5] and [6], respectively. This is evidenced by the shape of the absorbance curve.
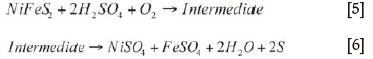
Table I shows the values of k obtained from solutions of all samples from the six sampling points, for the different metals.
The rate constants for the leaching reactions of the metals vary from 0.0424 to 0.0527 min-1 with corresponding half-lives of 16.39 to 13.15 minutes. This is consistent with the results shown in Figure 2, where it is evident that after about 90 minutes, the reaction had reached equilibrium. From Table II, it can be seen that the rate of leaching is in the order Fe2+ > Cu2+ > Ni2+ > Co2+. That is, Fe2+ was the fastest leaching metal ion followed by Cu2+, then Ni2+, with Co2+ the slowest to leach into solution. The rate of oxidation of these metal ions is expected to increase with increasing size of the metal ion (Jones and Atkins, 2000) because the smaller the metal ion the closer the outer electron is to the nucleus. Since the electrons are negatively charged they are therefore firmly held by the nucleus. Therefore, more energy is required for the loss of the electron from the outer orbital of a smaller metal ion. On the other hand, it is easier to remove an electron from the outer orbital of a relatively bigger metal ion as the outermost electron is less firmly held by the positive nucleus. Of the four metal ions, Fe2+ has the largest ionic radius, followed by Co2+, and Cu2+ the smallest. Their reactivities should therefore be of the order Fe2+ > Co2+ ≈ Ni2+ > Cu2+. Our results do not completely show this trend, which is an indication that other factors contribute to the rate of leaching of these metal ions.
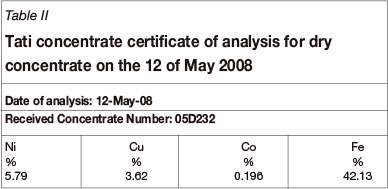
Apart from the size of the metal, the concentration of the species also has an effect on the rate of the redox reactions. Table II shows the concentration (in % w/w) of these metals in the May 2008 Tati dry concentrate that was used for this study. The data shows that the concentration of the metals in the concentrate was in the order Fe >>> Ni > Cu > Co. The much higher concentration of Fe2+, coupled with its largest size, explains why it had relatively much higher reactivity when compared to the other metal ions. It is also known that without chloride leaching, a passive layer forms around the particles that hinders or slows down the leaching of chalcopyrite (Lundström et al., 2005). Therefore, it is believed that chloride addition played a role in the rate of leaching, especially for Cu, as observed in this study.
Surge tank
In the surge tank, it seems there was little or no reactivity taking place, but only the dilution of the repulp liquor (Cu raffinate) by the incoming unleached slurry from the mills. This is evidenced by the dilution curves that were produced, as shown in Figure 4 for Fe. Similar curves were obtained for all the other metals studied.
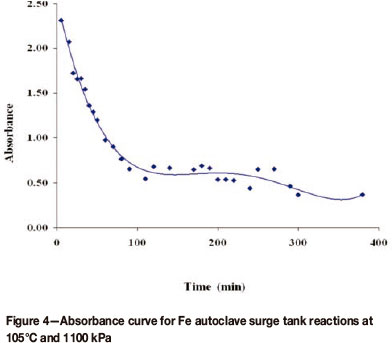
The plots of ln(A∞ - At) versus time were linear and from such plots, the pseudo-first-order rate constants were obtained as shown in Table II.
Autoclave compartments
Absorbance curves for compartment 1 shows a rather interesting pattern, as illustrated in Figure 5.
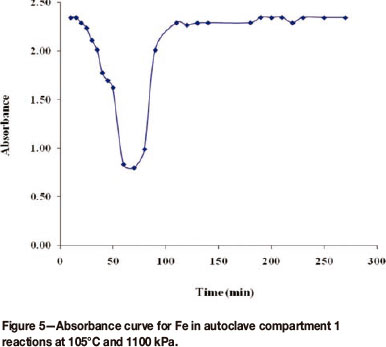
There was a decrease in absorbance for the first 60 or so minutes, which can be attributed to dilution, followed by a marked increase in absorbance until it levelled off after some time. This means that in compartment 1, dilution by the re-pulp liquor took place first, followed by leaching. The dilution and leaching rate constants for the metals under study in this compartment are shown in Table I.
The absorbance curves for compartments 2 and 3 showed a similar pattern to that of compartment 1, but with smaller minima. The dilution and leaching rate constants for these compartments are given in Table I. In compartment 4 and compartment 5 only leaching took place; there was no dilution and hence no dilution constants. Comparison of rates of leaching among the different compartments shows that the rate of leaching decreased on moving from compartment 1 to compartment 5 for all the metals studied.
The decrease from compartment 1 to compartment 4 is expected, in that in compartment 1 there are relatively little or no metal ions leached but more of the unleached metals locked in the solid phase, leading to an increase in the rate of leaching. In compartment 4 some of the leached metal ions from compartments 1, 2, and 3 have reached compartment 4, thus retarding the rate of leaching and hence the observed decrease in the rate of leaching in this compartment. This is consistent with what has been observed by other authors (Western Minerals Technology, 2004). If the rate of leaching in compartment 5 (and hence the autoclave discharge line) was the same as that of compartments 1 to 4, then it would be expected that compartment 5 (and hence the autoclave discharge line) will have the lowest rate constant. This was not the case, as k for the autoclave discharge is greater than for both compartments 1 and 2 (except for iron). This might be due to the fact that in compartment 5, there is little or no dilution due to the incoming unleached slurry, suggesting that only leaching took place, resulting in a marked increase in the rate of leaching in this compartment.
In almost all cases, dilution rate constants were higher than leaching rates constants. This could be due to the large volume of sulphuric acid in the system, i.e., high flow rate of 50 kg H2SO4 per ton. This means that the sulphuric acid added dilutes the copper raffinate faster that it leaches the metal ions.
Conclusion
Kinetic investigations on the leaching of Ni, Cu, Fe, and Co in a five-compartment autoclave showed that, for all the compartments studied, the rate of leaching was in the order Fe2+ > Cu2+ > Ni2+ > Co2+. This was attributed to the size of the metal ions studied, the concentration of the metal of interest, and presence of chloride ions in the circuit. The leaching rate decreased on moving from compartment 1 to compartment 4 for all the metals studied. Dilution by the repulp liquor played a major role in slowing down the leaching kinetics, especially in compartments 1 to 3.
Acknowledgement
The authors would like to thank Norilsk Nickel, Tati Nickel Mining Company, and the University of Botswana, Chemistry Department for technical and financial assistance.
References
Angove, J.E., Corrans, I.J., and Johnson, G.D. 1993. The recovery of nickel and gold from sulphide concentrates. Proceedings of the XVIII International Mineral Processing Congress, Sydney, Australia, 23-28 May 1993. AuslMM. pp. 1227-1231 [ Links ]
Corrans, I.J., Angove, J.E., and Johnson, G.D. 1995. The treatment of refractory coppergold ores using Activox® processing. Randol Gold Forum Perth '95 - Gold Metallaurgy & Environmental Management. Randol International Ltd,. pp. 221-224. [ Links ]
Johnson, G., Corrans, I., and Angove, J. 1993. The Activox® process for refractory gold ores. Proceedings of Randol Gold Forum 1993. Beaver Creek, Colorado, 7-9 September 1993. Randol International, Golden, Colorado. pp. 183-188. [ Links ]
Jones, L. and Atkins, P. 2000, Chemistry, Molecules, Matter and Change. 4th edn. Library of Congress cataloging-in-Publication data. Chapter 13. [ Links ]
Lundström, M., Aromaa, J., Forsén, O., HyvÄrinen, O., and Barker, M.H. 2005. Leaching of chalcopyrite in cupric chloride solution. Hydrometallurgy, vol. 77. pp. 89-95. [ Links ]
Nel, G.J. and van den Berg, D.A. 2009. Novel design aspects of the Tati Activox® Project ammonia recovery circuit. 5th Southern African Base Metals Conference, Kasana, Botswana, 27-31 July 2009. Southern African Institute of Mining and Metallurgy, Johannesburg. [ Links ]
Norilsk Process Technology. 2007. BMR Hydrometallurgical Plant Operating Manual, Plant Area 02, Leach. [ Links ]
Oyetunji, O.A., Paphane, B.D., and Becker, C.A.L. 2006. Kinetic studies on the reactions of some tetrakis(arylisocyanide)cobalt(II) complexes with pyridine in 2,2,2-trifluoroethanol. Transition Metal Chemistry, vol. 31. pp. 951-957. [ Links ]
Pilling, M.J. and Seakins, P.W. 2001, Reaction Kinetics. Oxford University Press, Great Britain. Chapter 2. [ Links ]
Welsh, S. and Barclay, L. 2006. Kinetic modelling of particles during Activox leaching-shrinking core/shrinking sphere. Technical note no. 6006. Norilsk Process Technology. Feb. 2006. [ Links ]
Welch, S. 2006. Kinetic modelling of pentlandide particles during Activox leaching-shrinking sphere. Technical note no. 6037. Norilsk Process Technology. Nov. 2006. http://web.chemistry.gatech.edu/~wilkinson/class_notes/CHEM_3111_6170/El electronic_spectra_of_TM_complexes.pdf [ Links ]
Western Minerals Technology. 2004. Hydrometallurgical demonstration plant process design criteria, leach chemistry and extent of reactions. p. 23. [ Links ]
Paper received Jul. 2011
Revised paper received Jul. 2012
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN 2225-6253.














