Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 no.5 Johannesburg Mai. 2013
Effect of dissolved precipitating ions on the settling characteristics of copper sulphide
M. Nduna; M. Rodriguez-Pascual; A.E. Lewis
University of Cape Town, Crystallization and Precipitation Unit, Cape Town, South Africa
SYNOPSIS
Surface properties of metal sulphides have a great significance in various areas of engineering and science, such as acid mine drainage, contaminant sorption, and metal separation. In various attempts at producing metal sulphide particles from synthetic solutions, prodigious quantities of nuclei that grow only to colloidal dimensions have been frequently reported. This copious nucleation is promoted by the high levels of supersaturation that characterize most precipitation reactions. Colloidal particle formation in precipitation-based separation processes results in sub-optimal solid-liquid separation, which is alleviated by the production of more highly crystalline particles or agglomerates. The current work approaches this challenge from an electrochemistry perspective, by measuring surface charge potential of precipitant particles during metal sulphide precipitation with respect to the concentration of metal sulphide lattice ions in solution. Electrophoresis was used to measure the metal sulphide particle zeta potential and the settling properties were obtained by performing settleability measurements using an Imhoff settling cone. A suspension of copper sulphide particles was precipitated from synthetic solutions of copper and sulphide ions at equimolar concentrations. Immediately after precipitation the copper sulphide particles had a zeta potential of -50 mV and a settleability of about 7 mℓ.ℓ-i. With the addition of copper ions the settleability increased by a factor of nearly three times and the zeta potential also increased to a maximum of -40 mV. A decrease in zeta potential to a minimum of -60 mV was observed after the addition of sulphide ions and this was associated with a settleability of 0 mℓ.ℓ-i.
Keywords: sulphide precipitation, settling, zeta potential, aggregation.
Introduction
Metal sulphide precipitation is a potential process for the removal of metal ions from industrial waste and acid mine drainage (AMD). Due to the relatively low solubilities of metal sulphides, effluent concentrations achieved can be less than 0.01 mg.l-1 in a tenth of the volume formed by the alternative conventional lime process (Huisman, et al., 2006; Peters, et al., 1984). Another potential application of the process is selective precipitation, which is possible due to the difference in solubility of the various metal sulphides. However, due to these low solubilities and the- great affinity between the reactant species, precipitation is inherently driven by high supersaturation. In conditions of high supersaturation, crystal growth rate is suppressed and nucleation rate favoured. Consequently, this leads to precipitant particles that grow only to colloidal dimensions, leading to poor sedimentation or filtration properties and a significantly lower practical removal efficiency than the predicted theoretical efficiency. Demopolous (2009) stated that the relationship between the processing conditions and product characteristics is a function of solid-liquid equilibria, reactor selection and design, precipitation kinetics, and colloid surface kinetics. In this study, the focus will be on investigating the effects of colloid-surface chemistry on the agglomeration properties of copper sulphide with the aim of enhancing sold-liquid separation, post-precipitation.
After precipitation, metal sulphide particles may develop charge as a result of one or more of the following mechanisms: ionization of surface groups, crystal defects leading to surface atoms that carry excess or deficient charge, and differential dissolution of surface lattice ions (Everett, 1988). When such a charged particle is immersed in an electrolyte solution, it is surrounded by ions of the opposite sign to balance the surface charge and this leads to the formation of what is commonly referred to as the electrical double layer (EDL) (Helmholtz, 1879). The EDL can be further divided into two regions: an inner layer of ions, which are closely associated with the charged surface, and an outer layer of ions that are loosely associated with the charged surface (Hiemenz, 1986). All the ions within the inner layer of the EDL are termed potential-determining ions (PDI). It is the diversity and nature of these ions that is important in predicting and understanding surface-mediated processes (Hunter, 1986). The interface between the inner and outer layer of the EDL is termed the shear plane and the potential at this plane is considered as the zeta potential of the particle (Shaw, 1970). The zeta potential of a particle can be quantified by micro-electrophoresis and is largely dependent on solution chemistry. Colloidal stability of a homogenous suspension is governed by the coulombic interactions between charged aqueous species and, therefore, is a function of zeta potential.
Deryagin and Landau (1941) and Verwey and Overbeek (1948) independently developed a quantitative theory, termed DLVO after the authors, in which the electrical stability of colloidal dispersions was treated in terms of the energy changes that take place when particles with an electrical double layer approach one another. The theory is based on the net interaction energy due to an overlap of electric double layers (repulsion) and van der Waals forces (attraction) in terms of interparticle distance. Particles that have a large zeta potential magnitude will experience large interparticle repulsions, and as such particles with a low zeta potential will experience relatively smaller interparticle repulsions. Therefore, it should follow that aggregation should be dominant in suspension systems in which the particles possess a low zeta potential magnitude, generally within the range -15 mV to +15 mV (Ottewill, 1982). This is also consistent with work conducted by Mokone and co-workers (2010), in which the authors explained the failure of precipitated copper sulphide particles to settle by the relatively high absolute value of their zeta potential, which was found between -30 mV and -45 mV.
The surface of sulphide minerals is highly reactive and begins to oxidize as soon as the mineral is in contact with water, if no particular precaution is taken. Fullston and co-workers (2006) reported that the zeta potential of sulphides is very sensitive to surface oxidation, with a change in magnitude of up to 80 mV between a non-oxidized and fully oxidized surface. Consequently, it is necessary to ensure minimal oxidation of the copper sulphide particles during the precipitation step. The isoelectric point of a homogenous suspension is the pH at which the respective particles possess a zeta potential of 0 mV. According to Healy and Moignard (1976), a 'pristine' and non-oxidized metal sulphide has an isoelectric point that lies at low pH values, close to that of elemental sulphur which has an isoelectric point of 1.6. As the extent of oxidation increases, the position of the isoelectric point shifts towards alkaline conditions and as such the relative position of the isoelectric point can be considered as a measure of oxidation. The investigation will include, firstly, determining the isoelectric point of the precipitated copper sulphide particles, in order to quantify the extent of oxidation, after which the effect of solution chemistry with respect to zeta potential and dissolved lattice ion concentration will be investigated.
Materials and methods
The investigation was divided in to two phases: firstly, the precipitation of copper sulphide particles, and secondly, modification of the solution chemistry with respect to copper and sulphide ion concentration. All the experiments were conducted at room temperature (approx. 25°C) and pressure, and solutions were made up to the desired concentrations using de-oxygenated and Millipore de-ionized water. Water was de-oxygenated prior to solution preparation by boiling the de-ionized water for at least 10 minutes and then allowing it to cool in a closed container. During solution preparation, oxygen contamination was avoided by sparging nitrogen whenever the vessel containing the solution was open to the atmosphere. Metal ion solutions were made up to a concentration of 500 mg.ℓ-1 and the sulphide ion solution concentration was made equimolar to the metal ion concentration. Both stock solutions were stored in 10 ℓ graduated vessels equipped with two ports, one for solution withdrawal and the other for nitrogen sparging. The precipitated metal sulphide particles were then suspended in metal ion and sulphide ion solutions that represented 3% and 5% of the respective synthetic stock solution. Ionic strength was maintained constant by using KCl, which was considered to be an indifferent electrolyte. Throughout the experiment, oxygen contamination was avoided by continuous sparging of nitrogen.
Micro-electrophoresis was used as an analytical technique to characterize the electrochemical nature of the copper sulphide particle surfaces. The zeta potential versus pH curve was produced using an MPT-2 Autotitrator in conjunction with the Malvern Zetasizer Nano. The autotitrator was connected to a sulphide-resistant pH probe, which was inserted into the sample measurement cell. Sample pH was adjusted by the addition of either 0.2 molar HCl or 0.2 molar NaOH. For a single sample, the suspension pH was changed from high to low pH with continuous zeta potential measurements at pH intervals of between 0.2 and 0.4, due to equipment limitations. All other zeta potential measurements were measured using the Malvern Zetasizer Nano, which uses the dynamic back-light scattering technique. All measurements were performed in triplicate, and experiments were also repeated three times. A settleable solids test was performed using an Imhoff cone mounted on a cone stand. The method involved adding a litre of a well-mixed test suspension into the Imhoff cone and leaving it to stand for 45 minutes. A rod was then gently run on the inside of the cone to loosen any solids clinging to the sides, and the suspension allowed to stand for a further 15 minutes. The results were measured in millimetres of settled solids per litre of suspension.
Metal sulphide particles were precipitated by pumping both Cu2+ and S2- into a 1 ℓ reactor at a combined residence time of 10 minutes. The reactor was kept at a constant pH of 6 and steady state was attained after approximately 30 minutes An overhead motor connected to a pitched blade stirrer was used for agitation and was operated at 650 r/min. After precipitation, the copper sulphide particles were kept in a closed vessel under nitrogen to avoid any possible oxygen contamination before they were suspended in different concentrations of Cu2+ ions or S2- ions. Zeta potential measurements of copper sulphide particles in the respective lattice ion solutions were conducted before a settleability test was performed on each of the samples.
Results and discussion
In order to characterize the zeta potential-pH behaviour of the copper sulphide particles, three independent precipitation experiments were carried out before the zeta potential of the respective particles was measured with respect to pH. The results are presented in Figure 1 as run 1, run 2, and run 3. All the three runs were between a pH range of 1.6 and 9, and the measured zeta potential was between a maximum of -8 mV and a minimum of -51.1 mV.
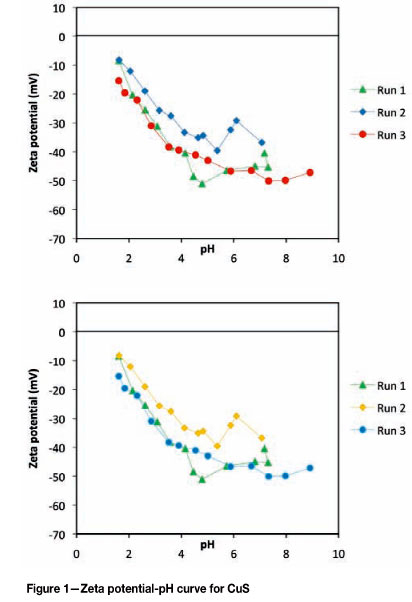
The maximum measured zeta potential values for all three experimental runs were consistently at a pH of 1.6. For runs 1 and 2 this corresponded to a zeta potential of -8.46 mV and -8.23 mV respectively, while a much lower value of -15.6 mV was observed for run 3. Runs 1 and 3 exhibited good reproducibility between a pH of 2 and 4, but a deviation of up to 8 mV was observed at a pH of 4.8, while the corresponding difference between runs 1 and 2 was much higher at 16.7 mV. In comparison to runs 1 and 3, run 2 consistently gave higher zeta potential values for corresponding pH values throughout the investigated pH range, and slight charge reversal behaviour was observed between a pH of 5 and 7. The general trend between runs 1 and 3 was similar and resulted in consistently lower zeta potential values with an increase in pH down to a minimum, after which a slight increase in zeta potential was observed.
The zeta potential-pH behaviour exhibited by the copper sulphide particles as shown in Figure 1 is characteristic of the behaviour of most transition metal sulphides. The observed decrease in zeta potential with an increase in pH is consistent with de-protonation of surface thiol groups (>S-H) and the rate of decrease is dependent on the respective surface acidity constants. At higher pH values, the reduction in the rate of zeta potential change with pH could be due to the saturation of surface hydroxyl groups (>Cu-OH). The consistently higher zeta potential values observed for experimental run 2 was most likely due to slight oxidation of the particles, which could have been caused by oxygen contamination during precipitation or sampling. The large deviation in zeta potential at a pH of 4.8 between run 1 and 3 was most likely due to measurement or sampling errors.
Electrokinetic studies on copper sulphide by Mokone and co-workers (2010) predicted an isoelectric point of less than 2, while Liu and Huang (1992) predicted an isoelectric point of less than 1. This is consistent with the isoelectric point of less than 1.6 as predicted in this study. Healy and Moignard (1976) showed that a low isoelectric point most likely indicates a slightly oxidized or un-oxidized metal sulphide surface. This suggests that the precipitated copper sulphide particles, in this study, can be considered as having undergone very minimal surface oxidation. Further to this, Dekkers and Schoonen (1994) stated that with significant oxidation leading to ion dissolution, charge reversal characteristics may be observed. This implies that the absence of charge reversal may also be consistent with limited oxidation.
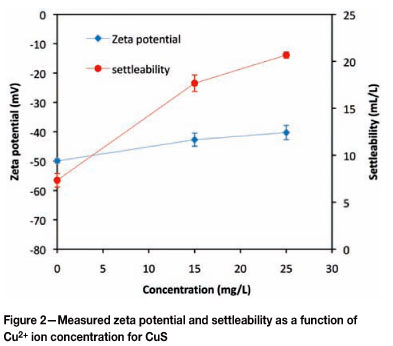
Immediately after precipitation, the copper sulphide particles had a zeta potential of -50 mV and a settleability of 7 m . -1. As the solution chemistry was modified by increasing the concentration of Cu2+ ions, an increase in the respective copper sulphide particle zeta potential and settleability was also observed. A suspension with an intermediate Cu2+ ion concentration of 15 mg. -1 resulted in particles that had a zeta potential of -42.7 mV and a settleability of about 18 m . -1. The associated rate of change- in zeta potential with relation to the Cu2+ ion concentration was about 0.46 mV for every milligram of copper ions added per litre. A maximum zeta potential of about -40 mV was attained when the copper sulphide particles were suspended in a solution with a concentration of 25 mg. ℓ-1 of Cu2+ ions, and these had a settleability of about 21 m .ℓ -1. The associated rate of change in zeta potential with relation to the Cu2+ ion concentration was 0.24 mV for every milligram per litre of copper ions added, about half the respective rate observed for the intermediate Cu2+ ion concentration. The maximum settleability observed, at a concentration of 25 mg. -1 of Cu2+ ions, was nearly three times more than that of the copper sulphide particles produced immediately after precipitation.
Figure 3 shows the effects of S2- ion concentration on both zeta potential and settleability of copper sulphide particles. The data points corresponding to 0 mg. -1 of S2-ions in Figure 3 are the same data points corresponding to 0 mg. -1 of Cu2+ ions in Figure 2.
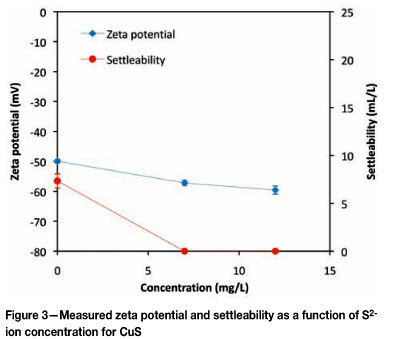
As the concentration of sulphide ions was increased, the respective zeta potential of the copper sulphide particles and the observed particle settleability decreased. The particles suspended in a solution with a S2- ion concentration of 7 mg.l-1 possessed a zeta potential of -57.2 mV. This translated to a decrease in zeta potential of about 7 mV and an associated particle settleability of 0 ml.l-1. A subsequent increase in the concentration of S2- ions led to a further decrease in the zeta potential of the copper sulphide particles and no change in settling characteristics. All the results- presented were reproducible, as shown by the error bars in Figure 2 and 3, which indicate standard error calculated from three experimental re-runs.
If a concentration increase is confined to the precipitating ions, specific adsorption of such ions on to the surface Bronsted sites can occur (Nicolau and Mernard, 1991). The net change in zeta potential would then depend on the ratio between the increase in metal ions and that of the sulphide ions. Dekkers and Schoonen (1994) summarized the effect of metal sulphide lattice ions on zeta potential as follows: an increase in metal ions results in an increase in the zeta potential of the particles and an increase in sulphide ions results in a decrease in the zeta potential of the metal sulphide particles. This is consistent with the results presented in Figure 2 and Figure 3, where an increase in zeta potential is observed upon suspending the particles in a solution of metal ions while a decrease in zeta potential is observed for particles suspended in a sulphide ion solution. The increase in zeta potential of copper sulphide particles in a metal ion solution may be due to a combination of factors. Metal ions are readily hydrolyzed in solution and form various aqueous species, whose concentration is pH-dependent. Up to a pH of about 6 the dominant copper- species in solution is the Cu2+ ion (Faur-Brasquet et al., 2002) and hence the dominant surface-PDI reactions up to this pH would be as described by Nicolau and Menard (1991), and defined by Equation [1]:
![]()
In a solution with a reduced Cu2+ ion concentration, the thiol group on the surface of the metal sulphide becomes deprotonated with an increase in pH. As shown in Figure 1, the zeta potential of un-oxidized copper sulphide particles is highly negative around a pH of 6. This is due mainly to the negative charge of the exposed sulphide ion on the thiol group (>S-). As the concentration of Cu2+ ions increases the negative charge on the metal sulphide surface is reduced because of the mechanism defined by the forward reaction of Equation [1]. According to Faur-Brasquet et al. (2002), copper hydroxyl species are stable at pH values of 6 and higher. Consequently such species may become adsorbed on to the surface of the metal sulphide, which may further increase the zeta potential of the copper sulphide particles.
When the concentration of S2- ions in solution is increased at a pH of 6, the dominant sulphide ion in solution is the HS- ion (Lewis, 2010). The HS- reacts with the metal hydroxyl group (>Cu-OH) on the surface of the metal sulphide as described by Nicolau and Menard (1991) and defined by Equation [2]:
![]()
The OH- species when integrated on the particle surface reduces the magnitude of the overall negative charge on a copper sulphide particle. This is mainly because the OH-species, which is less likely to lose a proton, occupies surface sites that may also be occupied by the HS- species, which readily loses protons in mildly acidic to alkaline conditions to expose a negative charge (>Cu-S-). The increase of sulphide ions in solution results in the substitution of surface OH-species with the HS- species, resulting in an increase in the density of potentially negative surface sites. This notion is consistent with the observed results presented in Figure 3.
Within the experimentally investigated conditions, the copper sulphide particles seem to obey the DLVO theory. When the zeta potential was increased to less negative values, an increase in settleability was observed. The increase in zeta potential reduced the magnitude of electrostatic forces, which are largely repulsive, resulting in the promotion of aggregation and hence improved settleability. When the zeta potential was decreased to less negative values, this translated to an increase in electrostatic forces, which led to a decrease in particle aggregation and hence a significant reduction in the observed settleability.
Conclusions
From the results presented it can be concluded that copper sulphide particles obey the DLVO theory and hence it is possible to induce or inhibit aggregation by altering the zeta potential of sulphide particles. This translates to an increase or a decrease in the electrostatic potential of the copper sulphide particles. By changing the concentration of copper sulphide lattice ions, either Cu2+ or S2- ions, it is possible to effectively change the surface properties of copper sulphide- particles and the associated zeta potential. A solution with a Cu2+ ion concentration of 15 mg.ℓ-1 was able to improve the settleability of precipitated copper sulphide particles by a factor of almost 2.5. This makes manipulating the zeta potential a viable option in improving the solid-liquid separation properties of metal sulphide precipitation processes.
References
Dekkers, J.M. and Schoonen, M.A.A. 1994. An electrokinetic study of synthetic greigite and pyrrhotite. Geochimca et Cosmochimica Acta, vol. 58, no. 19. pp. 4147-4153. [ Links ]
Demopolous, G.P. 2009. Aqueous precipitation and crystallization for the production of particulate solids with desired properties. Hydrometallurgy vol. 96, no. 3. pp. 199-214. [ Links ]
Deryagin, B.V. and Landau, L. 1941. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochimica, vol. 14. pp. 633-662. [ Links ]
Everett, D.H. 1988. Basic Principles of Colloid Science. Royal Society of Chemistry, London. [ Links ]
Faur-Brasquet, C., Reddad, Z., Kadirvelu, K., and Le Cloirec, P. 2002. [ Links ]
Modelling the adsorption of metal ions (Cu2+, Ni2+, Pb2+) onto ACCs using surface complexation models. Applied Surface Science, vol. 196, no. 1-4. pp. 356-365. [ Links ]
Fullston, D., Fornasiero, D. and Ralston, J. 1999. Zeta potential study of the oxidation of copper sulphide minerals. Colloids and Surfaces, vol. 146. pp. 113-121. [ Links ]
Healy, T.W. and Moignard, M.S. 1976. A review of electrokinetic studies of metal sulphides. Flotation, A.M. Gaudin Memorial Volume. vol. 1. Fuerstenau, M.C. (ed.). AIME, New York. p. 275. [ Links ]
Helmholtz, H. 1879. Studies of electrical interfaces. Annalen Der Physik Und Chemie, vol. 243. pp. 337-382. [ Links ]
Hiemenz, P.C. 1986. Principles of Colloid and Surface Chemistry. 2nd edn. Marcel Dekker, New York. [ Links ]
Huisman, J.L., Schouten, G., and Schultz, C. 2006. Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrometallurgy, vol. 83. pp. 106-113. [ Links ]
Hunter, R.J. 1986. Foundations of Colloid Science, vol. 1. Oxford University Press, New York. [ Links ]
Lewis, A.E. 2010. Review of metal sulphide precipitation. Hydrometallurgy, vol. 104. pp. 222-234. [ Links ]
Liu, J.C. and Huang, C.P. 1992. Electrokinetic characteristics of some metal sulphidewater interfaces. Langmuir, vol. 8, no. 7. pp. 1851-1856. [ Links ]
Mokone, T.P., Van Hille, RP., and Lewis, A.E. 2010. Effect of solution chemistry on particle characteristics during metal sulphide precipitation. Journal of Colloid and Interface Science, vol. 351. pp. 10-18. [ Links ]
Nicolau, Y.F. and Menard, J.C. 1991. An electrokinetic study of ZnS and CdS surface chemistry. Journal of Colloid and Interface Science, vol. 148, no. 2. p. 551. [ Links ]
Ottewill, R.H. 1982. Concentrated dispersions. Chemical Society Review Symposium Goodwin, J.W. (ed.). Royal Society of Colloidal Dispersions. pp. 197-217. [ Links ]
Peters, R.W., Ku, Y., and Batthacharyyya, D. 1984. Evaluation of recent treatmenttechniques for removal of heavy metals from industrial astewaters. AIChE Meeting. pp. 19-22. [ Links ]
Shaw, D.J. 1970. Introduction to colloid and surface chemistry. Butterworths, London. [ Links ]
Verwey, E.J. and Overbreek, J.T.G. 1948. Theory of the stability of lyophobic colloids. Elsevier, New York. [ Links ]
Paper received Apr. 2013;
Revised paper received Apr. 2013.
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN2225-6253.














