Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 n.5 Johannesburg May. 2013
Evaluation of processing options for the treatment of zinc sulphide concentrates at Skorpion Zinc
H.F. FulsI; J. PetersenII
ISkorpion Zinc, RoshPinah Namibia
IIUniversity of Cape Town, Rondebosh, Cape Town, South Africa
SYNOPSIS
Skorpion Zinc, an integrated zinc mine and refinery located in the south of Namibia, has a production capacity of 150 000 t/a of special high grade (SHG) zinc. The Skorpion zinc oxide resource will be depleted by 2017. Extensive exploration drilling for additional zinc oxides was conducted without success. With the abundant availability of zinc sulphide concentrates regionally (Black Mountain and Gamsberg in the Northern Cape or Rosh Pinah Zinc and new deposits in the Rosh Pinah region) the life of the operation can be extended by processing of zinc sulphide concentrates.
The leach kinetics of zinc sulphides is vastly slower than for oxides, preventing the processing of zinc sulphides in the existing process under current conditions, and Skorpion Zinc faces the challenge to identify a suitable zinc sulphide treatment process that can be integrated with the existing plant. Various zinc sulphide processing options were identified through an extensive literature search and non-viable options were eliminated. Mass and energy balances were established for potential options and an economic evaluation (including capital and operating cost estimation) was conducted to identify a suitable process.
It is concluded that ferric leaching of sphalerite in a sulphate medium under atmospheric or pressure leach conditions are suitable processes for Skorpion Zinc. These processes present advantages over all other zinc processing options when elemental sulphur is preferred as the final deportment for sulphide sulphur. Both these processes can be integrated into the existing refinery and will be able to process concentrates with a wide composition range.
Keywords: zinc processing, pressure leach, atmospheric leach, zinc sulphide.
Introduction
Skorpion Zinc, an integrated zinc mine and refinery located near Rosh Pinah in southern Namibia, produced its first metal in May 2003. The refinery has a production capacity of 150 000 t/a of special high grade (SHG) zinc (> 99.995% Zn). The orebody, a substantial oxide resource averaging 10.9% Zn, was discovered in 1976. The main minerals are sauconite (a zinc-bearing clay mineral), smithsonite (zinc carbonate), and hemimorphite (zinc silicate). The elevated silica levels and the presence of halides, together with challenges in upgrading the zinc ore, resulted in a delay in developing a processing route that includes direct leaching of the ore, purification via a solvent extraction process and conventional electrowinning to produce the final zinc product on site.
The Skorpion zinc oxide resource will be depleted by 2017. Extensive exploration drilling for additional zinc oxides was conducted without success. With the abundant availability of zinc sulphide concentrates regionally, from Black Mountain and Gamsberg in the Northern Cape or Rosh Pinah Zinc and new deposits in the Rosh Pinah region, consideration is given to convert the Skorpion Zinc refinery to process zinc sulphides. Concentrates can also be imported via Luderitz harbour, approximately 400 km from Skorpion Zinc. The leach kinetics of zinc sulphides is vastly slower than for oxides, preventing the processing of zinc sulphides in the existing process.
For sulphide ores, a concentrate containing zinc sulphide as sphalerite or marmatite is usually produced. The standard process for zinc sulphide concentrate treatment is the roast-leach-electrowinning (RLE) process. This process is highly efficient at converting the zinc in concentrate to SHG zinc metal by roasting to zinc oxide, acid leaching and purification, and electrolysis to zinc metal. The economics of this process are favourable when a market for sulphuric acid exists. There are a whole host of alternative hydrometallurgical processes for zinc (Filippou, 2004). Sphalerite and marmatite are highly leachable in a variety of solutions including sulphuric and chloride media. As is often the case in hydromet-allurgy, the sulphate route for zinc recovery has received the most commercial application with the Dynatec Zinc pressure leach, the Outotec (previously Outokumpu) atmospheric leach, and the Union Minière atmospheric leach installed at a number of sites. These processes essentially avoid the roasting step of the RLE by directly dissolving zinc from the sulphide mineral followed by purification and- electrolysis. These three processes have all been used to incrementally add to zinc production by being installed alongside existing RLE plants. The Dynatec pressure leach and the atmospheric leach processes have been installed in few instances as 'standalone' facilities.
The challenge for Skorpion Zinc is to identify a suitable zinc sulphide treatment process to be integrated with the existing plant. The selection of a process option will have to consider the cost of implementation, i.e. the time and capital required to convert the existing refinery to process zinc sulphides, including the impact on production output. Consideration also has to be given to various feed sources available, ensuring Skorpion Zinc remains a low-cost zinc producer in a volatile market and economic climate. Important considerations for a suitable process for Skorpion Zinc are:
► A process with more favorable economics will be preferred. This implies low operating and capital cost with high zinc recovery
► Low technical risk. A process that is commercially proven will be preferred against other options to reduce the risk to achieving design production and operating cost
► Schedule of implementation. The oxide resource will be depleted by 2017 and the new process must deliver the first zinc in 2017. Any process delivering zinc after this date will not be considered
► Skorpion Zinc is remotely located and cannot competitively deliver sulphuric acid to the market. A process that produces sulphuric acid will be considered as fatally flawed
► A low impact on safety and health of employees and low environmental impact will be preferred
► Skorpion Zinc is committed to a low carbon footprint and to reducing the use of natural resources. Processes with low energy and water consumption will be preferred.
This paper will present the process followed to identify a suitable zinc concentrate processing route for Skorpion Zinc. The initial screening and evaluation of potential processes will be discussed with the objective of eliminating unfavourable processes. The remaining process options will be further developed in order to establish technical integration with the existing refinery and to establish the capital and operating costs of each selected option. A suitable process will then be identified.
Screening of options
Figure 1 presents an overview of possible zinc concentrate processing routes compiled through a literature study. The pyrometallurgical processes involve the fuming of zinc, and condensation as zinc oxide that is further processed by an electrolytic process (dissolution, solution purification, and electrowinning) (Sannikov, 1998; Bartlett, 1985; Hughes et al., 2008; Buckett I., 1998). In the RLE process, zinc sulphide is oxidized to form a zinc oxide that is also processed by an electrolytic process (Filippou, 2004; Huggare et al., 1973; Svens et al., 2003). The pyrometallurgical and RLE processes produce SO2 gas as byproduct which is converted to sulphuric acid. Other processes involve the direct leaching and processing of zinc sulphides and can be further divided into atmospheric, pressure, or percolation leach options. From all the processes, RLE has been applied more widely and is the most popular process option in the 21st century. Several of the other processes have been established commercially and many hydrometallurgical routes proposed (but not developed commercially) to process more complex sulphides that could not be economically processed through the RLE or pyrometallurgical processes (Filippou, 2004). These processes will also be considered and evaluated.
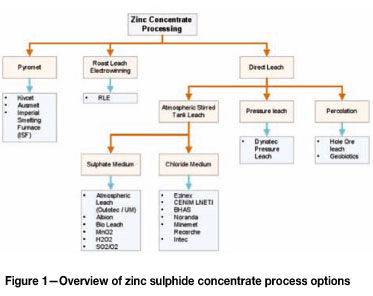
Pyrometallurgical and the RLE processes produce sulphuric acid as by-product. This acid is sold to a downstream user/local market for sulphuric acid. If no offset exists, it may result in stopping zinc production due to full sulphuric acid stock tanks or the acid will have to be neutralized and disposed of at high cost. Sulphuric acid is produced as a by-product in many refineries and a sensitive balance in the sulphuric acid market exists. Demand is also highly integrated with the world sulphur market. Sulphur is produced as a by-product in the oil refining industry at an oversupply and the long-term forecasts are that an oversupply in the sulphuric acid and sulphur market will remain (CRU). Large consumers for sulphuric acid are typically the phosphate industry and other hydrometallurgical processes. Within South Africa there is a good balance between sulphuric acid supply and demand. The uranium industry located near Swakopmund in Namibia is a net consumer of sulphuric acid. Skorpion zinc is very remotely located in the south of Namibia and will not be able to deliver sulphuric acid competitively to either of these two markets- due to high transport costs. A pyrometallurgical or RLE process at Skorpion will therefore increase the business risk. Having the primary business linked to another market, especially a by-product market, will be a disadvantage. The pyrometallurgical and RLE processes will therefore be discarded as options for Skorpion Zinc.
There are two options when the percolation leach process is considered. The first is the 'Geocoat' process from Geobiotics, where the zinc sulphide concentrate is coated on an inert substrate before being stacked and leached (Geobiotics). The leaching process takes place in a sulphate medium at low pH. Zinc sulphide is leached by ferric as an oxidant. The ferric is regenerated by oxygen and bacteria. Although it has not been commercialized to process zinc concentrates, the Geocoat process was applied at the Agnes Mine (Barbrook, Barberton, South Africa) to leach refractory gold concentrates. This mine has ceased operation. Some other projects are also under evaluation but not implemented yet. Harvey et al. (2002) reported 95% recovery from concentrates on a laboratory scale. This recovery is expected to be lower on a commercial scale due to the inherent challenges of solution distribution and percolation in heaps. It will be hard for this option, processing high-grade concentrates, to compete with alternative options with high recovery and slightly higher capital investment. Sulphide is converted to sulphate and elemental sulphur in this process. This option is discarded based on the limited commercial implementation and the long time associated with heap leach piloting and demonstration testwork.
The second heap bioleach option (Teck Cominco's HydroZinc process) is aimed at processing the mined ore directly (Lizama et al., 2003; Hunter et al., 2004). This option therefore cannot be compared directly to the other options discussed in this section as it does not include concentrate production. It is therefore compared to the crushing, milling, flotation, and leaching capital and operating costs. HydroZinc can be considered as an option for Skorpion Zinc only if a complete new mine is to be developed, where capital investment for a milling and concentrator plant can be offset with the capital of the heap leach and where the mine is located close to Skorpion. At present, potential feed sources are not close to Skorpion Zinc and trucking of a low-grade whole ore will not be viable due to the high transportation cost. This option is therefore eliminated.
The Dynatec pressure leach process involves the ferric leach of zinc sulphide at 150°C in a sulphate media. Ferric is regenerated by pure oxygen injected into the autoclave. It is a mature technology with successful implementation at various sites in the gold, platinum, and base metals industries. The Dynatec (previously Sherrit Gordon) pressure leach was first applied to treat zinc sulphide concentrates commercially in 1981 in the Cominco Zinc plant at Trail, British Columbia. The zinc pressure leaching plant included a single autoclave designed to treat 188 t/d of zinc concentrates (Ashman et al., 1990). It was an add-on to the existing RLE plant to expand capacity. Following Cominco, the pressure leach has been added to existing RLE plants to expand capacity at Kidd Creek (now Falconbridge/Xstrata) and a third plant at Ruhr Zinc in 1991 (Buban et al., 2000). The first two-stage pressure leach for zinc sulphide concentrates was commissioned during 1993 as a replacement for the RLE process at Hudson Bay Mining and Smelting Co., Ltd. (Barth et al.,- 1998). The two-stage pressure leach (standalone) option will be applicable to Skorpion. The two-stage process has significantly lower free acid in the pregnant leach solution (PLS) compared to the single-stage processes developed as add-ons to other processes where the residual sulphuric acid is consumed.
One of the primary advantages of the Dynatec process is that sulphur deports to elemental sulphur, which can be recovered or discarded with the residue. This is a critical consideration for Skorpion as discussed earlier. Furthermore, this process has a high zinc recovery with lower capital investment compared to the RLE process and has more flexibility to treat concentrates with iron, silica, and lead contents. It can tolerate a higher amount of pyrite and hence could positively impact zinc recovery at the concentrators. Silicates are essentially inert to the zinc pressure leach process and concentrates with high silica content are readily treated (unlike the RLE process).
The downside of this process is a higher energy cost (compared to the RLE process) as the energy to convert elemental sulphur to sulphuric acid is not harvested. It further requires pure oxygen for the process at high cost. Although proven technology, operating at high pressure increases safety risks and requires a higher level of attention during operation and maintenance. No fatal flaws associated with this process option are identified so far. Further development is required to establish the economics of this process.
The atmospheric leach processes can broadly be divided into two categories based on the leaching media - chloride and sulphate media. The leaching mechanisms in these media include acid leaching, alkaline leaching, and oxidative leaching. Various atmospheric leach processes in chloride media have been developed, including CENIM-LNETI (Figueiredo, 1993, 1995), EZINEX (Olper, 1998), Intec BHAS (Ricketts et al., 1989), Noranda (Allen et al., 2001), Minemet Recherche (Demarthe et al., 1978). The challenges to process complex sulphide ore economically and the need to achieve a residue-free process have driven the development of the various chloride-based processes. The Noranda process could not deliver a high-quality zinc product and recovery is low, and therefore the process never developed past the laboratory scale. Though the inventors claim reduced operating and capital cost for the INTEC process with environmental benefits, it has not been developed beyond a locked-cycle batch processing pilot plant. The same apples to the CENIM-LNETI, EZINEX, BHAS, and Minemet Recherche processes. The material of construction is also costly in a chloride leach environment at high temperature (50-100°C), and is a disadvantage compared to sulphate medium processes. The chloride media processes might have some advantages when complex sulphides have to be processed to maximize recovery of all valuable elements. Due to the time required to develop these processes to commercial scale and the fact that Skorpion Zinc will process a typical zinc concentrate, none of these processes will be further considered.
To simplify the discussion of atmospheric leach of zinc sulphide in sulphate media, three categories will be considered. First the development of atmospheric leach process with oxygen injection (Outotec, Union Minière, and Albion); second, the bioleach process; and thirdly, all other processes developed to laboratory stage.
Outotec (previously Outokumpu) and Union Minière independently developed the atmospheric leach process, initially as an add-on process to existing RLE operations (Filippou, 2004; Van Put et al., 1999; Fugleberg, 1998). This process involves the ferric leach of zinc sulphide just below 100°C. Ferric is regenerated by pure oxygen injected into the reactors. The key to the success of the atmospheric leach processes is the specially designed reactors to enhance the oxygen dispersion in the slurry. The technology can be considered mature with low technical risk for implementation. This process has a high recovery with the primary advantage of producing elemental sulphur and not sulphuric acid. It has the perceived advantage over pressure leaching of lower capital and maintenance costs due to operation at atmospheric pressure. This process also requires an oxygen supply and has a net energy requirement, similar to the pressure leach process. As it operates below the melting point of sulphur, it has the advantage over pressure leach by not requiring a surfactant (such as lignosulphonate or quebracho). There appear to be no fatal flaws associated with the atmospheric leach process, and the economics compared to other potential options for this process will have to be evaluated.
The Albion process depends on a fine grind to achieve fast reaction kinetics to achieve reasonable economics (Hourn et al., 1996, 1999). This process is commercially applied on zinc sulphide concentrates at the San Juan de Neiva and Nordenham zinc refineries. Fine milling technology is being applied successfully elsewhere in the minerals processing industry, and the Albion atmospheric leach processes for zinc sulphides have been proven to be successful. Sphalerite is a sulphide mineral that leaches relatively easily compared to other sulphide minerals, which enables it to be leached under atmospheric conditions, as proven by Outotec and Union Minière. Test work has also proven that the kinetics is enhanced by a finer particle size distribution. The solution chemistry of the Albion process is very similar to the pressure and atmospheric leach processes. The decisive question to be answered is whether the additional capital and operating costs for fine grinding can be offset by the reduced capital cost and operating costs of atmospheric leach by speeding up kinetics. There is no fundamental flaw, but also no clear disadvantage to this process. Further development is required to establish true capital and operating costs more accurately.
Although the stirred tank bioleach process has not been commercialized in zinc processing, it is commercially applied in the gold industry and can therefore not merely be rejected (Filippou, 2004; Steemson, 1994; Nilsson, 1996; Sandstrom and Peterson, 1997; Sandstrom, Sundkvist, and Peterson, 1977). The role of the bacteria in the leaching process is to oxidize the ferrous to ferric for the leach of sphalerite. Oxygen is provided by sparging air through the slurry, eliminating the need for an expensive pure oxygen supply. Sulphur-oxidizing bacteria, also present, oxidize the product sulphur layer formed on the sphalerite surfaces. The downside of the bioleach process is the long residence time required. The sphalerite dissolution is surface reaction rate-controlled (Harvey et al., 1993; Souza et al., 2007) and the kinetics is strongly driven by reaction temperatures. This explains the long residence times required for bioleach, which typically takes place at temperatures of 45-65°C compared to the atmospheric leach process at temperatures of 95-100°C. This further explains the reduction in residence time required- when extreme thermophilic organisms are used, allowing operation at temperatures of 65°C compared to the MIM bioleach process that operates at about 45°C with moderate thermophilic bacteria. In the order of 3-5 days' residence time is required to leach a zinc concentrate with a particle size distribution of 90% passing 45 µm. At 15% solids concentration and with the long residence time of the bioleach process, the reactor volume for the sphalerite leach is much higher than for the atmospheric leach. Work by Sandstrom and Peterson (1997) indicated that residence time can be shortened by fine grinding (90% passing 20 µm). This compares to the feed size distribution of the Albion process, although the residence time is much longer than for the Albion process due to the low leach temperatures. Xstrata (Hourn et al., 2005) conducted an economic trade-off study for a refractory gold ore where the Albion, pressure leach, and bacterial oxidation options were compared. The bacterial oxidation process presented the highest capital and operating cost.
Pyrite, the most noble sulphide mineral in the electrochemical series, also starts to oxidize under bioleaching conditions. All pyrrhotite is dissolved and some pyrite, resulting in a total iron dissolution between 30% and 90%, depending on the mineralogy, temperature, redox potential, and leach conditions. At 65°C and the redox potentials necessary to achieve high sphalerite dissolution, between 60% and 90% iron is dissolved, depending on the residence times (Sandstrom and Peterson, 1997). This is significantly higher than in the atmospheric, pressure and Albion leach processes, putting bioleaching in a less advantageous position compared to the other processes, as a higher amount of iron has to be removed from solution (most likely as a jarosite due to the high iron content and relatively lower acidic conditions - jarosite is the least-preferred iron residue).
This process has a further disadvantage compared to the pressure, atmospheric, and Albion processes due to the oxidation of sulphur to sulphate. The additional sulphate is removed from solution by jarosite formation and the remaining sulphates have to be precipitated.
Zinc tenor from the bioleach process (approx. 30 gi) is not suitable for direct electrowinning, which require zinc tenors in solution around 150-160 gjℓ. A solvent extraction (SX) step is therefore required to upgrade the solution for electrowinning. Although no capital cost will be required if this process is chosen for Skorpion Zinc, as a SX plant is already installed, it has higher sulphuric acid and neutralization costs compared to conventional purification processes and hence is at a disadvantage compared to the pressure and atmospheric leach processes.
As this process has a disadvantage relative to the pressure, atmospheric, and Albion leach processes, it will not be further considered as an option for Skorpion Zinc. It has a potential application in the gold industry where the objective is to dissolve the pyrite and other sulphide gangue to liberate the gold.
The processes discussed in the previous paragraphs involve the leaching of sphalerite under oxidizing conditions in an acidic ferric solution. Other oxidative leach processes in sulphate media have also been tested at laboratory scale:
► Mixture of SO2 and O2
► Pyrolusite (MnO2)
► Hydrogen peroxide.
Adams et al. (1981) describe the leaching of metal sulphide concentrates at atmospheric pressure using SO2/O2 mixtures. Their laboratory testwork indicates that SO2/O2 mixtures of appropriate composition rapidly leach a variety of zinc concentrates at atmospheric pressure in aqueous solution containing sulphuric acid and ferrous iron. The work indicates that an oxygen to sulphur dioxide ratio of 1:1.5 is required for the reaction to occur. The oxygen consumption will therefore be fairly high if the gas is not recycled. This process has not been commercialized, with the main challenge being scale-up due to the large gas-flow volumes required.
Filippou (2004) cited work by many others regarding the use of manganese dioxide as an oxidant for zinc sulphide. MnO2 is a very effective oxidant at low pH and can leach zinc directly from zinc sulphides as follow:
MnO2 + ZnS + 2H2SO4 - ZnSO4 + MnSO4 + SO
Electrowinning of zinc from solutions containing high levels of Mn is virtually impossible. An economical method to separate the Mn from the zinc-rich solution needs to be found for this process to be successful. An electrolytic process for removal of manganese from zinc electrowinning solutions was commercially used at Sulfacid S.A. zinc plant in Argentina. The disadvantages included the high costs due to low current efficiencies, the need to cool electrolyte to 15°C in the manganese removal step, and high misting in the cellhouse due to the need to sparge air into the cells.
The leaching of sphalerite concentrate by means of hydrogen peroxide (H2O2) as an oxidant in sulphuric acid solutions was examined by Pecina et al. (2008) and Aydogan (2006). Hydrogen peroxide is a strong oxidizer that forms water during the oxidation of sulphides. An increase in sulphuric acid, hydrogen peroxide concentration, and temperature and decreasing particle size increased the leaching rate. Test work indicates a recovery of between 60% and 80%. With this low recovery and high cost of hydrogen peroxide it is unlikely that this process will be economical.
None of these processes (SO2/O2, MnO2, H2O2) have been developed commercially and will not be further considered due to the time it will take for development and the associated technical risks.
To summarize, the favoured processes for Skorpion Zinc are the ones not producing sulphuric acid as by-product, and which have been developed to commercial scale. The pyrometallurgical process and electrolytic (RLE) processes have been eliminated as they produce sulphuric acid as byproduct. Hydrometallurgical processes in chloride media and processes involving oxidants other than ferric in a sulphide media have been discarded based on the lack of development past laboratory scale. Bioleach processes have been rejected due to dilute sulphuric acid produced and the need for a subsequent SX process to upgrade the zinc tenor to a level suitable for electrowinning. Hydrometallurgical processes that involve ferric leaching in sulphate media (pressure leach, atmospheric leach and the Albion process) are favoured and will be considered for further economic evaluation.
Detailed analysis of favourable options
The selected process options will be further developed in order to establish technical integration with the existing refinery and to conduct an economic assessment. For the economical assessment a capital and operating cost estimate was established. A flow sheet and mass and energy balance for each process was developed and capital costs were estimated by establishment of mechanical equipment costs by Hatch Africa using their database of recent completed projects (post-2001). Total capital cost was then estimated from mechanical cost by factoring of other costs (electrical, civil, instrumentation, project management, etc.). Accuracy of the capital cost estimate is ± 30%. Operating costs were established by factoring maintenance cost, compiling organizational structure with personnel requirement, and costing of reagents by actual reagent prices at Skorpion Zinc and volumes from the mass and energy balance.
Although the commercial pressure and atmospheric acid leach processes do not require a solvent extraction stage to upgrade the zinc, there could be a potential benefit from the SX plant as it provides an additional buffer to impurities for the zinc electrowinning stage. As a SX circuit is installed as part of the oxide process, no additional capital would be required. The impact of incorporating SX with the proposed processes was evaluated by incorporating it as part of the pressure leach process for comparison. The pressure leach can be substituted with any of the atmospheric leach or Albion processes.
The flow sheet developed for each option will first be presented in this section, followed by a discussion on the economics of the various processes.
Atmospheric leach
The process flow of the atmospheric leach is presented in Figure 2. Zinc sulphide concentrate is fed to a two-stage countercurrent atmospheric leach circuit at a rate of 34 t/h. The two- stage process will be applied in order to reduce the residual free acid concentration in the PLS (reducing neutralization cost). PLS solution from the low-acid leach (LAL) contains approx. 10 g/ℓ free acid and total soluble iron of approx. 8 g/ℓ by careful control of acid and oxygen addition. Solids from the first stage are transferred to the high-acid leach (HAL) stage where it is subjected to a high-concentration sulphuric acid leach for another 15 hours close to 100°C. Reaction enthalpy is sufficient to maintain temperature without any external heating required (Lahtinen et al., 2005). Solution from the HAL contains 13-14 g/ℓ Fe, approx. 48 g/ℓ H2SO4, and approx. 134 g/ℓ zinc. Solids concentration is around 9% in both leach stages and oxygen is sparged into the leach trains to oxidize ferrous to ferric. The solids from the HAL contain elemental sulphur, some unreacted sulphides (pyrite and chalcopyrite), and insoluble sulphates (lead sulphate). A total residence time of 30 hours has been used for this process (even though 24 hours is recommended by Lahtinen, 2005). This is to ensure the high zinc recovery of 98% is achieved at a concentrate feed particle size distribution of 90% passing 45 µm.
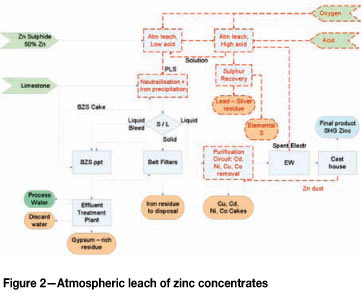
The HAL solution is thickened and can be further processed to recover the elemental sulphur from a flotation and melting processes. The PLS solution, with zinc tenors suitable for electrowinning (156 g/ℓ) is sent to sequential purification steps. Iron is first removed in an iron precipitation process. Any type of iron removal (jarosite, goethite, or haematite) can be applied (Haakana et al., 2008). It was assumed for this study that a goethite removal step will be used due to the availability of basic zinc sulphate (BZS) from the BZS process and limestone as neutralization reagents. Limestone is present in the oxide resource and can be mined and milled to 90% passing 45 µm. Iron will be precipitated at a pH between 3 and 4 with oxygen sparging to oxidize the ferrous to ferric. This process typically takes place at 60-90°C. The iron content in solution is reduced to 6-10 mg/ℓ, suitable for electrowinning. Solid precipitated iron residue is filtered and washed with a zinc-free solution, provided by the BZS plant.
Solution from the iron removal process is further purified by a cold and hot conventional zinc cementation process to remove Cu, Cd, Ni, Co, and rare earths before processed in the zinc electrowinning plant where zinc is plated.
New equipment that is costed is presented by the dotted (red) lines in Figure 2. As specially designed reactors are required for the concentrate atmospheric leach process, the existing agitated leach tanks cannot be utilized and will be replaced. An oxygen plant will be constructed to supply oxygen for the atmospheric leach and iron precipitation processes.
The sulphuric acid consumption will be 4.6 t/h, about 10% of the design capacity of the existing sulphur-burning acid plant, well below its turn-down capacity. This study assumes the buy-in of sulphuric acid and a trade-off study to construct a low-capacity acid plant is recommended for a later stage. Offloading and storage facilities exist at Skorpion.
Iron removal, especially the goethite removal described in the literature, is typically conducted at temperatures between 60°C and 95°C. This is primarily to achieve high reaction rates and reduce equipment sizing. In most of the processes the feed solution is already at high temperatures (from the leach processes) and no additional heating is required. In the proposed atmospheric leach circuit the LAL solution feeding the precipitation circuit will be around 70-78°C. The existing neutralization equipment is designed for a temperature up to 55°C and is therefore not suitable for the neutralization process without modification. The reaction kinetics of the goethite iron removal process at 55°C (existing equipment limitation) need to be better understood to assess whether the existing equipment could provide sufficient residence time for this process at lower temperatures, before a final conclusion on existing equipment can be made. Alternatively, equipment modifications must be made to allow operating at higher temperatures. For the purpose of this study it was assumed that the existing equipment will not be used, and- the cost of a new iron removal circuit is included. An opportunity exists to reduce capital with upgrading of the existing equipment.
The mass balance indicates that the flow rate of iron-free PLS to the purification circuit is 177 m3/h with a copper tenor of 0.9 g/i. The existing copper cementation circuit is designed for a flow rate of approx. 350 m3/h and solution tenor of 0.88 g/i. This indicates that the existing copper removal circuit is sufficient. However, the existing hot purification circuit (for Ni and Co removal) is sized for 70 m3/h and will have to be upgraded to process the targeted 177 m3/h.
The iron residue to be disposed of is estimated to be 22 dry tons per hour, with significantly lower filtration fluxes than the current residue. The existing residue filtration is modular and able to process 195 t/h material. The existing system will be able to process the projected iron residue. The BZS circuit will provide wash solution for the iron residue as well as the lead-silver residue. The projected required feed rate to the BZS circuit is around 61 m3/h, within the turndown ratio of the BZS plant. No capital is therefore required for modification of the BZS circuit.
Iron and the lead-silver residues may contain heavy metals that could be mobilized during high rainfall years. For the purpose of this study it has been assumed that all residues will be disposed on lined facilities. The selection of the goethite process in preference to the jarosite will require a smaller footprint and reduce disposal capital requirements.
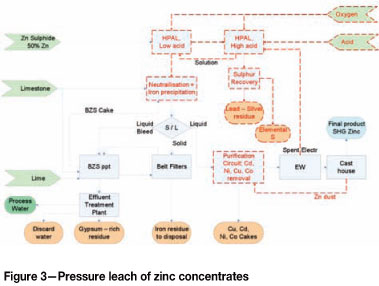
Dynatec pressure leach
The Dynatec pressure leach (150°C) process flow sheet (Figure 3) is very similar to the atmospheric leach process flow sheet. The leach process will also be conducted in a two-stage countercurrent flow to achieve a lower final acid concentration of 8 g/i and 2 g/i dissolved iron. Zinc tenor in the LAL discharge slurry is 160 g/i, sufficient for electrowinning without upgrading. The HAL solution contains 30 g/i acid and 15 g/i dissolved iron (Barth et al., 1998). The process chemistry of pressure leach and atmospheric leach are very similar, with the main differences in reaction kinetics and the stability regions of jarosites. At elevated temperatures, the reaction kinetics are significantly faster (Harvey et al., 1993; Butinelli et al., 1992). At 150°C an overall residence time of around 2 hours (approx. 1 h per stage) is sufficient to achieve 98% dissolution of zinc sulphides. The downside is that special equipment (autoclave) is required to operate at elevated temperatures to keep water in the liquid state. This equipment requires high capital and skills for maintenance. The other downside of high-pressure leaching is the scaling that takes place in the autoclaves, resulting in bi-annual shuts to clean the autoclaves. To achieve high availabilities, typically a third standby autoclave is constructed and piped in to be used either as a LAL or HAL stage. This further increases capital cost. The advantage though is a smaller footprint and associated civil and structural costs. Heat required for the pressure leach process and iron removal step is supplied by the exothermic reactions. Sulphur is also in the molten state, requiring the use of surfactants to prevent the coating of unreacted zinc sulphides.
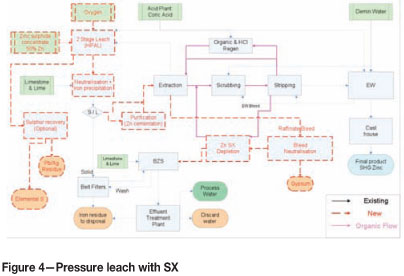
Pressure leach combined with SX
The leach section of this process is the same pressure leach as described earlier, with the difference that spent electrolyte is not returned to the autoclave, but rather the raffinate from the extraction circuit. PLS from the leach section will go through an iron removal step and residues through a wash step before disposed on a lined tailings facility.
As in the Skorpion Oxide process, Cu, Cd, Ni, and Co will build up in the leach circuit and a removal step needs to be introduced. This can be achieved by either a bleed circuit or by processing the total PLS stream, depending on the allowable copper tenor in PLS. When a certain threshold copper tenor in PLS is reached, there is some carry-over to the electrolyte via entrainment. For this study, it was- assumed that the threshold copper concentration is 0.9 g/ℓ and therefore the full PLS stream will have to be processed through the impurity removal process.
Zinc-rich solution (14 5 g/ℓ Zn) from the iron removal/purification circuit is extracted in the solvent extraction, leaving a raffinate with zinc tenor of approx. 55.4 g/i. The expected percentage extraction is scaled up linearly from the current operating circuit. Isotherms for extraction at elevated temperatures and PLS zinc tenors will have to be developed if a more accurate estimate is required. For the purpose of this study the linear scale-up would be sufficient. Zinc is stripped from the loaded electrolyte and recovered in a conventional zinc electrowinning process. Electrolyte has to be bled from the electrowinning process to prevent a build-up of impurities in the closed circuit electrolyte solution. Lost electrolyte is replaced with deminer-alized water and chemically pure sulphuric acid.
Excess water and sulphuric acid present in the circuit, mainly from the electrolyte bleed, scrubbing stages, and acid requirement for the organic regeneration necessitates a liquid bleed from the process. This is done by scavenging zinc from a raffinate bleed stream in a zinc depletion SX and a final polishing step in the BZS circuit. From here the solution is bled from the circuit. Excess sulphuric acid is removed by a neutralization step in front of the zinc SX depletion step. Acid consumption could potentially be reduced by 50% by using alternative technology (like resin or evaporative regeneration) to regenerate the hydrochloric acid used to remove iron from the stripped organic. The acid requirement to replace bled electrolyte will remain.
Acid consumption of this circuit at 8 t/h is roughly 18% of the design of the existing acid plant. This is significantly below the turn down ratio of 30-40%. Significant modifications to the acid plant will have to be made in order to produce sulphuric acid. It has been assumed for this study that the acid will be bought in.
The only benefit from this circuit is to provide an additional buffer for impurities, allowing production of SHG zinc more consistently. This will, however, add much more complexity to the design and operations of the plant. The high sulphuric acid consumption and associated neutralization and tailings disposal costs make this option very unattractive.
Albion leach
The process flow of the Albion process is presented in Figure 5. It is very similar to the atmospheric leach process, with the difference of a milling step before the leach. Received concentrate (90% passing 45 µm) is milled to approx. 90% passing 20 µηι or smaller. The objective is to improve kinetics and reduce residence time in the leaching process, hence reducing the capital required. Leach reactors will also require less power input compared to the Outotec reactors as a more conventional stirred tank reactor with hydrofoil impellers with lower power input can be used (Outotec reactors have a high aspect ratio with an internal draft tube. The reactors have a high internal circulation rate with a specially designed impeller to promote oxygen dispersion, resulting in high power input). Oxygen is sparged into the tanks use a supersonic oxygen injection lance in combination with the hydrofoil impellers to achieve the required mass transfer and leaching rate.
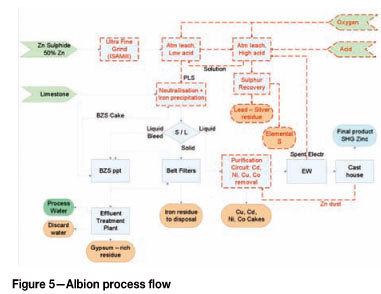
The leaching process for the Albion process is very similar to the atmospheric leach process and the same reactions and rates have been assumed for the Albion process. The only difference is the required residence time that is reduced to 24 hours to achieve the same reaction extents.
When considering the fit with existing infrastructure and equipment, the same arguments as for the atmospheric leach prevail for the Albion leach. The existing oxide ore ball mill is not suitable for the ultra-fine grinding and will have to be replaced with the ISAmill technology provided by Xstrata Technology.
Operating cost
The reagent consumptions of the various options are presented in Table I.
Sulphuric acid consumption for the atmospheric leach is twice the consumption of the pressure leach. This is a direct result of the leach conditions selected for the two processes -specifically the outlet acid, ferrous and ferric concentrations. Reaction extent for both processes was the same with the exception of jarosite that was allowed to form in the pressure leach process, also impacting on the acid consumption. Jarosite precipitation liberates acid and reduces the overall acid consumption. The process outlet tenors for sulphuric acid, ferric, and ferrous chosen were sourced from the literature. Extensive research and development has been conducted and published on the pressure leach process since it was introduced in the 1980s. The atmospheric leach process was commercially introduced only in the late 1990s with comparatively less research and actual data published. Lathtinen et al. (2005) report that the conditions in the atmospheric leach can be manipulated to achieve a wide range of acid tenors and redox potentials. This can very well be achieved by variation of the oxygen injection and slurry density in the leach. However, for the purpose of this study, parameter selection was based on published data. The mass and energy balance indicates that reagent consumption is very sensitive to the actual leaching kinetics and conditions and the form of iron precipitation.
The acid consumption for the pressure leach with SX is six times the acid consumption for the pressure leach alone, a major disadvantage of this process option. This is primarily a result of the water requirement of the washing stages of SX and the electrolyte bleed that consumes acid. Demineralized water is used to top up the water losses in the SX and EW circuits-further increasing the cost of this option.
The limestone requirement for the pressure leach with the SX follows the high acid consumption, as the refinery bleed stream (to balance Mg tenors and total solution) has a higher acid tenor, which has to be neutralized. Likewise, the higher limestone requirement for the atmospheric leach compared to the pressure leach is due to higher sulphuric acid tenor in the solution to the neutralization section.
Oxygen consumption for all the options in the mass balance was assumed to be the same. The number reported in Table I is based on stoichiometric requirement. In reality, the oxygen conversion efficiency will differ between the atmospheric and pressure leach. Although Lathtinen et al. (2005) mentioned that oxygen consumption in the pressure leach is higher than in the atmospheric leach, it is expected that the oxygen will have lower efficiency due to the ambient operating pressure and the higher volumes of the atmospheric reactors. It has been assumed that the oxygen conversion rate for the atmospheric leach will be 87% of the conversion rate in the pressure leach reactors. This has been accounted for in the data presented in Table II.
Although it is reported in the literature that both the atmospheric and pressure leach processes operate autothermally due to the heat released by the exothermic reactions, there is a steam requirement in the sulphur melting process, in the hot purification circuit, and to pre-heat solutions to the leach sections. The pressure leach with SX has an additional heat requirement due to the cooling of PLS before the SX circuit, which results in a heating requirement of the raffinate before it is fed back to the pressure leach circuit.
The calculated operating costs for each option are presented in Table II. Again, the operating cost is expressed as a percentage of the total cost of the atmospheric leach. Around 55% of the cost is locked in fixed costs, which include maintenance, labour, management, administration, and support services. These were based on actual operating cost at Skorpion Zinc.
For the Albion process, the grinding cost presented includes energy consumption (assuming 25 kWh/t energy requirement), grinding media cost, and maintenance cost. Maintenance cost includes wear items like the shell, disks, and other wear items.
Zinc dust consumption is indicated as zero. Zinc dust will be produced internally and circulated back to the purification circuit, with no requirement to buy in zinc dust.
The major contributors to the variable cost are electricity, sulphuric acid, limestone, and consumables (anodes and cathodes). The reagent cost estimate is based on consumption rates presented and recent quotations from suppliers.
Pressure leach has the lowest operating cost, roughly 8% below that of atmospheric leach. This is driven primarily by the assumptions made for the leaching conditions and iron precipitation. Maintenance cost is expected to be lower for the atmospheric leach equipment as specialized components and brick lining are not required for the atmospheric reactors. This is reflected in the factored maintenance cost estimate (2% of capital cost).
The operating cost comparison between these options is highly sensitive to the process chemistry with regards to redox potential (ratio of ferric to ferrous), acidity of leach stages, and the precipitation of iron during the leach process. The atmospheric and pressure leach options compare equally favourably. All three processes have high zinc recovery, which is very dependent on variability in concentrate size distribution. The atmospheric leach and pressure leach are preferred over the Albion leach process as the latter has not been applied commercially. Even though the ultrafine grind is well established commercially, leaching of a fine grind in conventional stirred tank reactors has not been applied commercially on zinc concentrates and will increase risk and require further development work. The Albion process will therefore not be further considered. However, a fine particle size distribution might be required by the atmospheric leach processes, depending on the mineralogy and reaction kinetics. In such a case the ISAmill technology could be considered.
If the economics are considered for the pressure and atmospheric leach, at a zinc price of US$2000 per ton zinc produced and a life of 20 years, the net present values of both options are equal. The pressure leach appears to be more attractive due to the higher number of standalone processes commercialized and a lower sensitivity to the size distribution of the zinc concentrates. However, the atmospheric leach option cannot be discarded based on the findings of this study. The atmospheric leach process operating cost is highly sensitive to the leach parameters (residual ferric, ferrous, and acid concentration) selected for the mass and energy balances. Atmospheric leach is relatively new and little information on the process is published. In addition, some uncertainty does exist on the concentrate particle size distribution required for the atmospheric leach process, as conflicting data is reported in the literature. Comparative- testwork by Buban et al. (2000) indicates that a finer grind is required to obtain high recovery under atmospheric leach conditions, while Lahtinen et al. (2005) report that a concentrate particle size of approx. 90% passing 44 µm is sufficient to obtain high recovery within 24-30 hours.
Capital cost
The atmospheric leach capital cost estimate includes 12 reactors (7 for LAL and 5 for HAL), each with 5 m diameter and 25 m height to achieve the 30 hours residence time. The atmospheric leach tanks are equipped with 90 kW and 75 kW motors for the LAL and HAL respectively. Some uncertainty exists on the mechanical costs for the atmospheric leach reactors. The reactors of Outotec and Union Minière are both specialized and proprietary equipment and actual costs not available in the open domain. Costs were estimated based on the reactor dimensions and assumption on the material of construction. It is known that the agitators require a high power input and the above power requirements were assumed. One spare reactor was included for each of the HAL and LAL stages.
For the capital cost estimate of the pressure leach circuits (with and without SX), three carbon steel brick-lined autoclaves were included - two operational and a third as standby. The standby autoclave can be tied into the circuit into the place of any one of the HAL or LAL autoclaves. The standby autoclave is to ensure a plant availability of 98% is achieved despite the maintenance stops required for brick lining and de-scaling. The size required to achieve a retention time of 60 minutes per autoclave is estimated at 4.5 m diameter and 22.5 m length, 4 compartments with 5 agitators (45 kW each).
Capital cost for the Albion process included an ISAmill for the fine grind duty and atmospheric leach tanks. The M3000 model ISAmill with 1.1 MW drive was selected for this process. It was sized based on a conservative power consumption estimate of 35 kWh/t to grind from a P90 of 45 µm to 20 µm. A percentage runtime of 85% was assumed and is considered conservative. The capital estimate for the Albion atmospheric leach circuit was based on a total of 11 reactors (including 2 standby) and power duties of 72 kW and 60 kW drives for the LAL and HAL respectively. A conventional reactor with length to diameter ratio of 1:1 was assumed for the costing.
The zinc concentrate handling, iron removal, and hot and cold purification circuits were all assumed to have the same duty for all the options. Table III presents the duty of all various unit operations for the capital cost estimates.
Capital is expressed as a percentage of the total cost of the atmospheric leach for discussion purposes, and is presented in Table IV. The total capital cost for the pressure leach is around 22% more than the atmospheric leach. This is a result of the higher capital cost of the autoclaves and auxiliary equipment compared to the atmospheric leach reactors. Integration of the SX circuit with the conventional process route has a further disadvantage of capital cost, around 41% higher than the atmospheric leach option. This is owing to the additional equipment required to neutralize the bleed stream and the additional zinc depletion SX circuit. Capital required for the Albion process is very similar to that of the atmospheric leach process. The additional capital for the ISAmill is offset by a 20% reduction in the residence time and hence cost of the leach reactors. The marginal reduction in agitation power also contributes to the offset.
Buban et al. (2000) compared only the leach sections of the pressure and the atmospheric processes. In their estimate, the pressure leach capital cost is between 1.08 and 1.25 times higher than the atmospheric leach. This study estimate the pressure leach capital cost at 1.75 times the atmospheric leach (leach sections only), higher than the estimate by Buban et al. They compared the cost of a single-stage atmospheric and pressure leach processes, whereas this study investigates two-stage leach processes and includes a standby autoclave, which Buban et al. have not considered. The assumptions made on the cost estimate of the proprietary equipment supplied by Outotec and the potential impact on the results must also be noted.
Conclusion
A review of all zinc producing processes against the- requirements of Skorpion Zinc eliminated firstly all the pyrometallurgical processes and the RLE process due to the production of sulphuric acid as by-product. The alkaline, chloride medium, and oxidative leach processes in sulphide medium (excluding ferric leach) are eliminated because they have not been commercialized and would require additional development work. This will add additional technical risk to the project and extend past the deadline to have processing capacity installed. The bioleach processes have been discarded as they produces a dilute acid and the PLS tenors from the bioleach is not suitable for direct electrowinning. A solvent extraction step would be required to upgrade the zinc tenors, increasing operating cost significantly.
Potential options identified for Skorpion Zinc for economic evaluation were the atmospheric leach, pressure leach, and the Albion processes. The enhanced kinetics of dissolving sphalerite with ferric ions in sulphate solutions is well known, and is influenced primarily by temperature, particle size, ferric concentration, and oxygen concentration, (Dreisinger et al., 1990; Harvey et al., 1993; Baldwin et al., 1995; Demopoulos et al., 1999; Souza et al., 2007). The various ferric leach processes developed aimed to benefit from these various parameters, with temperature as one of the primary drivers. The capital and operating costs for the three options are summarized in Table V, expressed as a percentage of the atmospheric leach option.
The option to consider inclusion of the solvent extraction circuit to reduce risk of 'off-spec' zinc production cannot be justified. There is no benefit or reduction in capital cost if the SX is included and more importantly, it increases operating cost by approximately 40% against the option with the lowest operating cost.
This study therefore concludes that ferric leaching of sphalerite in a sulphate medium under atmospheric or pressure leach conditions are both suitable for Skorpion Zinc. These processes presents advantages over all other zinc processing options when elemental sulphur is preferred as the final deportment for sulphide sulphur. Both these processes can be integrated into the existing refinery and will be able to process concentrates with a wide composition range.
References
Adams, R.W. and Matthew, I.G. 1981. Leaching of metal sulfide concentrates at atmospheric pressure using SO2/O2 mixtures. Proceedings of the Australasian Institute of Mining and Metallurgy, vol. 280. pp. 41-53. [ Links ]
Allen, C., Kondos, P., Payant, S., Van Weert, G., and Van Sandwijk, A. 2001. Production of zinc oxide from complex sulfide concentrates using chloride processing. PCT World Patent WO 01/25497, 12 April 2001. [ Links ]
Ashman, D.W. and Jankola, W.A. 1990. Recent experience with zinc pressure leaching at Cominco. Lead-Zinc '90. Mackey, T.S. and Prengaman, R.D. (eds.). TMS, Warrendale, PA. pp. 253-275. [ Links ]
Aydogan, S. 2006. Dissolution kinetics of sphalerite with hydrogen peroxide in sulfuric acid medium. Chemical Engineering Journal, vol. 123. pp. 65-70. [ Links ]
Baldwin, S.A., Demopoulos, G.P., and Papangelakis, V.G. 1995. Mathematical modeling of the zinc pressure leach process. Metallurgical Transactions, vol. 26B. pp. 1035-1047. [ Links ]
Barth, T.R., Hairu, A.T.C., and Meier, T.P. 1998. The operation of the HBM&S zinc pressure leach plant. Zinc and Lead Processing. Dutrizac, J.E., Gonzalez, J.A., Bolton, G.L., and Hancock, P. (eds.). The Metallurgical Society of CIM. pp. 811-823. [ Links ]
Bartlett, R.W. 1985. Economic trade-offs in increasing revenue from a massive complex sulfide deposit-Caribou. Complex Sulfides, Processing of Ores, Concentrates and Byproducts. Zunkel, A.D., Boorman, R.S., Morris, A.E., and Wesely, R.J. (eds.). TMS-AIME, Warrendale, PA. pp. 783-799. [ Links ]
Buban, K.R., Collins, M.J., Masters, I.M., and Trytten, L.C. 2000. Comparison of direct pressure leaching with atmospheric leaching of zinc concentrates. Lead-Zinc 2000. The Minerals, Metals and Materials Society. pp. 727-738. [ Links ]
Buckett, G.A. and Sinclair, R.J. 1998. The BUKA Zinc Process: a 21st century standard. Proceedings of Zinc and Lead Processing. Dutrizac, J.E. et al. (eds.). CIM. pp. 579-596. [ Links ]
Buttinelli, D., Lavecchia, R., Pochetti, F., Geveci, A., Guresin, N., and Topkaya, Y. 1992. Leaching by ferric sulphate of raw and concentrated copper-zinc complex sulfide ores. International Journal of Mineral Processing, vol. 36. pp. 245-257. [ Links ]
CRU. http://www.crugroup.com [ Links ]
Demarthe, J. M. and Georgeaux, A. 1978. Hydrometallurgical treatment of complex sulfides. Complex Metallurgy '78. Jones, M.J. (ed.). Institute of Materials, Minerals and Mining, London. pp. 113-120. [ Links ]
Demopoulos, G.P. and Baldwin, S.A. 1999. Stoichemometric and kinetic effects on the pressure leaching of zinc concentrates. TMS Annual Meeting, 28 Feb. - 4 Mar. 1999. The Minerals, Metals & Materials Society. [ Links ]
Dreisinger, D.B., Peters, E., Talaba, M., DeGraaf, K.B., Owusu, G., and Swiniarski, R. 1990. The kinetics of the Sherrit Gordon Zinc Pressure Leach Process. Lead-Zinc '90. Mackey, T.S. and Prengaman, R.D. TMS, Warrendale, PA. pp. 313-333. [ Links ]
Figueiredo, J.M., Nováis, A.Q., Limpo Gill, J., and Amer, S. 1995. The CENIM-LNETI Process for zinc recovery from complex sulfides. Zinc & Lead '95. Mining and Materials Processing, Japan. pp. 151-160. [ Links ]
Figueiredo, J.M., Nováis, A.Q., Limpo Gill,J., and ámer, S. 1993. The CENIM-LNETI Process for zinc recovery from complex sulfides. Proceedings of Zinc '93. Australasian Institute of Mining and Metallurgy. pp. 333-339. [ Links ]
Filippou, D. 2004. Innovative hydrometallurgical processes for the primary processing of zinc. Mineral Processing and Extractive Metallurgy Review, vol. 25. pp. 205-252. [ Links ]
Fugleberg, S. and Jarvinen, A. 1998. Method for leaching zinc concentrate in atmospheric conditions. PCT World Patent WO98/06879, 19 February 1998. [ Links ]
Geobiotics. http://www.geobiotics.com [ Links ]
GlencoreXstrata. http://www.albionprocess.com. [ Links ]
Haakana, T., Saxen, B., Lahtinen, L., and Takala, H. 2008. Outotec direct leaching application in China. Lead and Zinc 2008. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 69-84. [ Links ]
Harvey, T.J., Yen, W.T., and Paterson, J G. 1993. A kinetic investigation into the pressure oxidation of sphalerite from a complex concentrate. Minerals Engineering, vol. 6, no. 8-10. pp. 949-967. [ Links ]
Harvey, T.J., Van der Merwe, W., and Afewu, K. 2002. The application of the GeoBiotics GEOCOAT bio-oxidation technology for the treatment of sphalerite at Kumba Resources' Rosh Pinah Mine. Bio & Hydrometallurgy '02, Cape Town, 13-15 March 2002. [ Links ]
Hourn, M.M., Rohner, P., Bartsch, P., and Ngoviky, K. 2005. Benefits of using the Albion process for a North Queensland project and a case study of capital and operating cost benefits versus bacterial oxidation and pressure oxidation. Albion Process. www.albionprocess.com/publications.html [ Links ]
Hourn, M.M., Turner, D.W., and Holzberger, I.R. 1996. Atmospheric mineral leaching process. PCT World Patent WO 96/29439, 26 September 1996. [ Links ]
Hourn, M.M., Turner, D.W., and Holzberger, I.R. 1999. Atmospheric mineral leaching process. US Patent 5,993,635, 30 November 1999. [ Links ]
Huggare, T.L., Ojanen, A., and Kuivala, A. 1973. How zinc concentrates are processed at the Outokumpu zinc plant in Kokkola. International Symposium on Hydrometallurgy, Chicago, Illinois, 25 February-I March, 1973. Evans, D.J.I. and Shoemaker, R.S. (eds.). pp. 770-805. [ Links ]
Hughes, S., Reuter, M.A., Baxter, R., and Kaye, A. 2008. Ausmelt technology for lead and zinc processing. Lead & Zinc 2008. International Symposium on Lead and Zinc Processing, Durban, South Africa, 25-29 February 2008. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 147-162. [ Links ]
Hunter, C.J., Williams, T.L., Purkiss, S.A.R., Cheung, W., Connors, E., and Gilder, R.D. 2004. Bacterial oxidation of sulfide ores and concentrates. US Patent uS2004/0206208 A1. [ Links ]
Intec. http://www.intec.com.au [ Links ]
Lahtinen, M. and Takala, H. 2005. Atmospheric zinc concentrate leaching technology of Outokumpu. Lead & Zinc '05. Proceedings of the International Symposium on Lead and Zinc Processing, Kyoto, Japan, 17-19 October 2005. pp. 803-816. [ Links ]
Lizama, H.M., Harlamovs, J.R., Belange, S., and Brienne, S.H.R. 2003. The Teck Cominco HydroZinc Process. Proceedings of Hydrometallurgy 2003. [ Links ]
Young, C.A. et al. (eds). TMS, Warrendale PA. pp. 1503-1516. [ Links ]
Nilsson, L., Peterson, S., and Sandstrom, A. 1996. A new process for zinc recovery from bacterial leach solutions. Scandinavian Journal of Metallurgy, vol. 25. pp. 161-171. [ Links ]
Olper, M. 1998. The EZINEX process - five years of development from bench scale to a commercial plant. Zinc and Lead Processing. Dutrizac, J.E.,
Gonzalez, J.A., Bolton, G.L., and Hancock, P. (eds.). The Metallurgical Society of CIM. pp. 545-560.
Pecina, T., Franco, T., Castillo, P., and Orrantia, E. 2008. Leaching of a zinc concentrate in H2SO4 solutions containing H2O2 and complexing agents. [ Links ]
Hydrometallurgy 2008. Proceedings of the Sixth International Symposium on Hydrometallurgy, Phoenix, Arizona, 17-20 August 2008. Society for Mining, Metallurgy & Exploration. [ Links ]
Ricketts, N.J., Johnson, R.D., and Smith, G.D.J. 1989. Hydrometallurgical treatment of Cu-Pb-Zn sulfide concentrates. Extractive Metallurgy '89. IMM, London. pp. 705-720. [ Links ]
Sandstrom, A. and Peterson, S. 1997. Bioleaching of a complex sulfide ore with moderate thermophilic and extreme thermophilic microorganisms. Hydrometallurgy, vol. 46. pp. 181-190. [ Links ]
Sandstrom, A., Sundkvist, J-E., and Peterson, S. 1997. Bio-oxidation of a complex zinc sulfide ore: a study performed in continuous bench- and pilot scale. Proceedings of the International Biohydrometallurgy Symposium, IBS97-BIOMINE97. Australia Mineral Foundation. pp. M.1.1.1-M.1.1.11. [ Links ]
Sannikov, Y.I., Liamina, M.A., Shumskij, V.A., Grinin, Y.A., and Radashin, M.V. 1998. A physical and chemical description of the Kivcet lead flash smelting process. CIM Bulletin, vol. 91, no. 1022, July/August. [ Links ]
Souza, A.D., Pina, P.B., LeAo, V.A., Silva, C.A., and Siqueira, P.F. 2007. The leaching kinetics of a zinc sulfide concentrate in acid ferric sulphate. Hydrometallurgy, vol. 89. pp. 72-81. [ Links ]
Steemson, M.L, Sheehan, G.J., Winborne, D.A., and Wong, F.S. 1994. An integrated bioleach/solvent extraction process for zinc metal production from zinc concentrates. PCD World Patent WO 94/28184, 24 May 1994. [ Links ]
Svens,K., Kerstiens, B., AND Runkel, K. 2003. Recent experiences with modern zinc processing technology. Erzmetal, vol. 56. pp. 94-103. [ Links ]
Van Put, J.W., Terwinghe, F.M.I.G., and De Nys, T.S.A. 1999. Process for the extraction of zinc from sulfide concentrates. uS patent 5, 858, 315. 12 January 1999. [ Links ]
Paper received Jan. 2013
Revised paper received May 2013
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN2225-6253.














