Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 no.5 Johannesburg may. 2013
The recovery of copper from a pregnant sulphuric acid bioleach solution with developmental resin Dow XUS43605
C.J. LiebenbergI; C. DorflingI; S.M. BradshawI; G.A. AkdoganI; J.J. EksteenII
IDepartment of Process Engineering, University of Stellenbosch, Matieland, South Africa
IIDepartment of Metallurgical Engineering, Western Australia School of Mines, Curtin University, Perth, Western Australia
SYNOPSIS
This paper focuses on the application of ion exchange technology for the recovery of copper from a leach solution originating from a heap bioleach in which base metals are leached from a low-grade ore that bears platinum group metals. Screening tests indicated that Dow XUS43605 has high selectivity for copper over the other metals in the solution, namely nickel, iron, cobalt, zinc, manganese, and aluminium. Batch adsorption kinetic experiments showed that copper adsorption equilibrium is attained at a fast rate. The kinetics of adsorption increased as the temperature was increased from 25°C to 60°C due to the decrease in solution viscosity and the subsequent improved intra-particle mass diffusion. Single-component Langmuir and Freundlich isotherm models were fitted to the batch copper adsorption equilibrium data, and a maximum copper capacity of 26 g/ℓ was observed for Dow XUS43605. The effects of flow rate, temperature, pH, and initial metal concentration on the dynamic recovery of copper were investigated in fixed-bed columns, and it was determined that temperature and flow rate had the most significant impacts on the loading of copper on the resin at copper breakthrough. A 36% increase in copper loading at breakthrough was observed when the temperature was increased from 25°C to 60°C. Finally, it was determined that a split elution is possible by using different concentrations of H2SO4 to first elute co-loaded nickel from the resin, followed by the elution of copper.
Keywords: copper, hydrometallurgy, ion exchange.
Introduction
Background
Mwase (2009) and Mwase et al. (2012) provided details of potential hydrometallurgical process routes for the recovery of base metals and precious metals from a low-grade platinum group metal (PGM) orebody. These routes entail the leaching of base metals (BMs) from the ore with 10 g/ℓ sulphuric acid and thermophilic micro-organisms (primarily Sulfolobus metallicus and Metallosphaera sedula) in a primary heap bioleach, followed by a second-stage cyanide heap leach to recover the PGM values from the solid residue of the primary heap bioleach (Mwase, 2009; Mwase et al., 2012). Since the bioleach is catalysed by iron-oxidizing bacteria, ferrous iron has to be fed to the heap at a concentration of 2 g/ℓ in addition to the sulphuric acid and micro-organisms. The addition of iron to facilitate the leaching process should be minimized by, for example, recovering and recycling of the iron from the leach solution. The main objective of this study was, however, to investigate the usage of ion exchange resins for the recovery of copper from the leach solution.
The initial BM and gangue element head grades of the solid material typically fed to the leaching process are shown in Table I and the percentage metal dissolution achieved after 30 days, as reported by Mwase (2009), is shown in Table II. The resulting bioleach solution exits the primary heap at 65°C with a pH in the range of 0.8-1.2, and with the following composition: 276 ppm copper, 389 ppm nickel, 2133 ppm total iron (ferrous and ferric), 13.11 ppm cobalt, 6.11 ppm zinc, 310 ppm aluminium, 119 ppm magnesium, and less than 2 ppm other impurities. The objective of this study was to select an appropriate resin for the recovery of copper from the bioleach solution and to conduct a series of batch kinetic and equilibrium tests, as well as continuous tests, on the selected resin in order to evaluate its performance under different operating conditions.
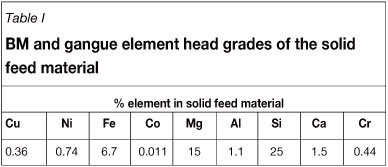
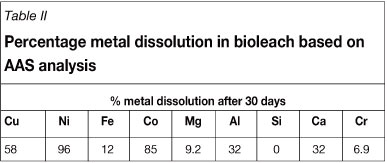
Despite the low percentage iron dissolution achieved in the leach, the iron concentration in the leach liquor is high, as a result of the concentration at which it is fed to the bioleach. At this high concentration, iron (in the ferrous or ferric oxidation state) could potentially be- detrimental to the downstream recovery of copper, nickel, and cobalt with chelating ion exchange. The solution therefore has to be purified of iron prior to using ion exchange for metals recovery from the solution.
A popular way of purifying aqueous solutions from iron without significant losses of target metals such as copper, nickel, cobalt, and zinc is by hydroxide precipitation (Habashi, 1990; Monhemius, 1981; Mouton et al., 2007). This is realizable only if iron is in the ferric oxidation state, as it precipitates at a pH of 2. Ferrous iron, on the other hand, precipitates at a higher pH than copper, which precipitates at a pH of 6, and in the same pH range as nickel and cobalt, which is between 7 and 9 (Monhemius, 1981). It is therefore recommended that the ferrous iron in the bioleach solution first be oxidized using a mixture of SO2 and air, after which it could be precipitated from the solution using hydroxide precipitation (Ho and Quan, 2007; Zhang and Muir, 2010).
Theory
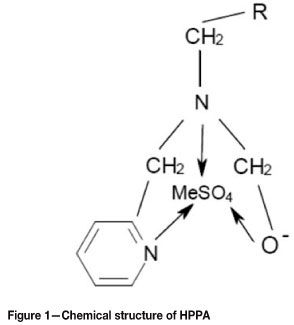
Dow XUS43605 consists of hydroxypropylpicolylamine (HPPA) ligand supported by a polymeric backbone of styrene (St) cross-linked with divinylbenzene (DB). The chemical structure of HPPA is shown in Figure 1 (Marston and Rodgers, 2010).
In metal adsorption with Dow XUS43605, the metal ion acts as a Lewis acid as it accepts electrons, and the chelating ligand acts as a Lewis base as it donates electrons (Pearson, 1963; Irving and Williams, 1953; Sirola, 2009). The selectivity of an ion exchanger depends on various factors, including the conditions of polymerization (which in turn affects the number and distribution of functional groups attached to the resin structure itself and its affinity towards the metal ions in the solution), the solution composition, solution pH, as well as the type of ligand attached to the polymeric backbone and its affinity towards the metal ions in the solution (Nicol, 2003; Sirola, 2009). Amongst these, the ligand-metal interaction has the greatest influence on a resin's selectivity (Sirola, 2009).
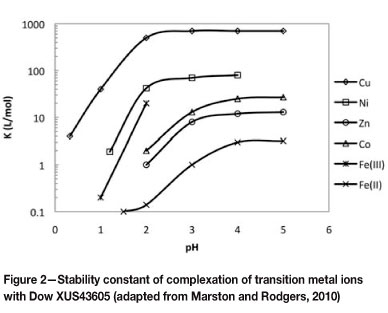
A wide variety of resin functionalities that could be used for copper adsorption is available, amongst which bis-picoly-lamine (Bis-PA), iminodiacetic acid (IDA), and aminophos-phonic (AP) are the most common (Grinstead, 1984; Mendes and Martins, 2005; Rosato et al., 1984; Zainol and Nicol, 2010). Problems encountered with the aforementioned resins include, firstly, extremely high copper selectivity and hence the difficulty of eluting copper from these resins, and secondly, poor iron rejection (Grinstead, 1984; Mendes and Martins, 2004). In the case of Bis-PA type resins, copper cannot be stripped using sulphuric acid; rather, an ammoniacal solution has to be used. This led to the development of Dow XUS43605, which has a lower copper complexation constant than Bis-PA type resins and better iron rejection than IDA and AP type resins (Marston and Rodgers, 2010). The stability constants for the complexation of various transition metal ions with Dow XUS43605 as a function of pH are shown in Figure 2 (Marston and Rodgers, 2010).
The use of Dow XUS43605 for the recovery of copper from solvent extraction raffinate was demonstrated in a pilot-scale study by Rodgers and Marston (2010). A solution containing 150 ppm copper, 600 ppm iron, 55 ppm cobalt, 45 ppm nickel, 250 ppm zinc, 800 ppm manganese, and 7.7 g/ℓ magnesium was passed through a fixed-bed column of Dow- XUS43605 at a flow rate of 20 bed volumes (BV) per hour and a temperature of 25°C. The copper loading achieved on Dow XUS43605 at copper breakthrough was in the order of 12 g/ℓ which was well below the maximum copper capacity of 35 g/ℓ (Dow, 2011). This resulted from the high flow rate at which the column was operated, and it is believed that this loading could be improved by changing some of the operating conditions at which the dynamic adsorption process was carried out.
To the authors' knowledge, the study by Rodgers and Marston (2010) is the only literature available that discusses the recovery of copper with Dow XUS43605; no kinetic or equilibrium data could be found for this resin. The effects of operating conditions such as solution pH, temperature, and flow rate on the copper adsorption were furthermore not fully elucidated by Rodgers and Marston (2010), which motivated the investigation of these parameters in the current study.
Experimental
Materials
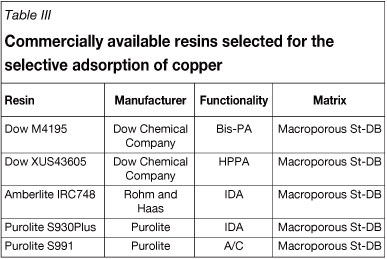
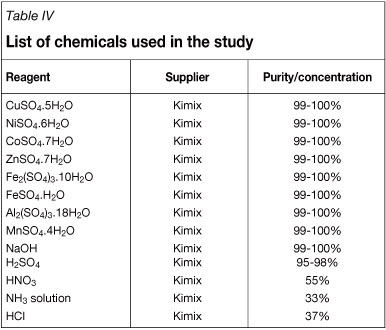
The chelating ion exchange resin used in this work, Dow XUS43605, is a macroporous polystyrene divinylbenzene resin with HPPA functionality and a uniform particle size of 320 µΐη. The resin was supplied by Dow Chemical Company. For comparative reasons, Dow M4195 (bis-PA functionality), Amberlite IRC748, Purolite S930 (IDA functionality), and Purolite S991 (amine/carboxylic functionality) were also tested for the recovery of copper from the bioleach solution. A summary of the resins that were investigated is provided in Table III. Reagents that were used to prepare synthetic bioleach solutions and eluting agents, along with the respective suppliers and reagent purities, are listed in Table IV.
Methods
Pre-treatment of resins
The resins listed in Table III were pre-conditioned by packing each resin in a glass column with a sintered bottom and washing with 10 BV of distilled water at a flow rate of 10 BV per hour to remove solid particles and impurities originating from the polymerization process. Each resin was then washed- with 10 BV of 1M H2SO4 at a rate of 5 BV per hour to ensure that it was in the protonated form, after which it was washed with 10 BV of distilled water once more to remove excess acid. After removal of the resin from the column and removal of excess water through filtration, it was stored at room temperature in the container that it was supplied in.
Preparation of synthetic bioleach solution
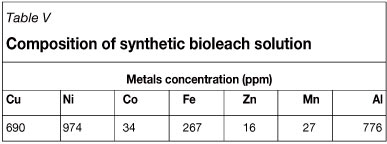
A bioleach solution that would be encountered in practice would be more concentrated than the solution obtained in the single-pass, pilot-scale leaching demonstration by Mwase et al. (2010). The main reason for this is that some of the effluent from the heap and from downstream operations would be recycled to the heap. For this reason, the approximation of the composition of the bioleach liquor that would be encountered in practice is difficult. Synthetic bioleach solution was prepared by assuming that copper would have leached to the same extent as nickel and cobalt if the test were allowed to continue for another 60 days. Based on the foregoing discussion, it was furthermore assumed that 98% of the iron in the solution could be removed from the solution using selective oxidation with SO2/air followed by precipitation and filtration prior to ion exchange. The expected average composition of the bioleach solution to be treated in the ion exchange process was therefore estimated to be as shown in Table V, and the synthetic bioleach solution was prepared accordingly. This was done by dissolving the correct amounts of the respective metal salts listed in Table IV in distilled water, except for ferric iron, which was dissolved in 1M H2SO4 before it was added to the solution containing the other metals.
Resin screening
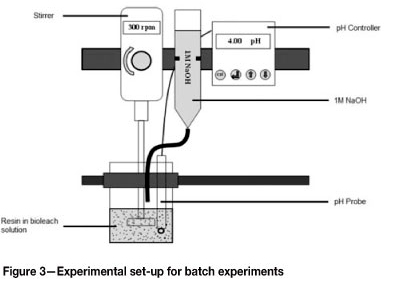
The resins listed in Table III were screened based on their suitability to adsorb copper selectively from the bioleach solution. This was achieved by contacting the various resins with synthetic bioleach solution in stirred beakers at a ratio of 1:50 (i.e. 5 mL tapped-settled resin contacted with 250 mℓ leach solution) and initial solution pH values of 3 and 4 for 48 hours to ensure that equilibrium had been attained. At the end of the 48 hour period, samples were withdrawn from the beakers for analysis. The experimental set-up that was used is illustrated in Figure 3.
Batch kinetic and equilibrium adsorption
Pre-treated Dow XUS43605 was contacted with synthetic bioleach solution at a ratio of 1:50 in continuously stirred beakers in batch kinetic experiments. The pH of the solution was maintained at the desired value by adding 1M NaOH dropwise to the beaker using an automatic pH controller supplied by Eutec Instruments. Small aliquots of solution were withdrawn from the beakers at various time intervals over a 24 hour period.
In the batch equilibrium adsorption experiments, Dow XUS43605 was contacted with the synthetic bioleach solution in ratios ranging from 1:100 to 1:5. For each ratio tested, a sample of the solution phase was taken after 48 hours.
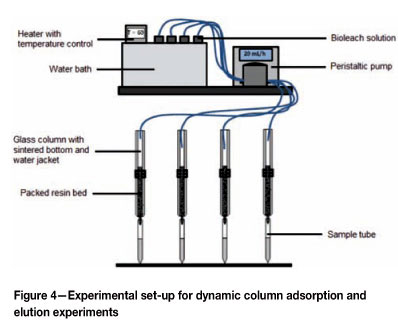
Dynamic column adsorption
10 mL of tapped-settled resin was placed in glass columns. The columns were 300 mm long and 12 mm in diameter, and equipped with sintered bottoms and water jackets. The synthetic bioleach solution was pumped from an overhead reservoir into the columns using a peristaltic pump. The temperature of the overhead reservoir and the temperature of the column were controlled at the desired set-point. Samples of the barren solution were collected at the bottom of the each column. A schematic illustration of the experimental set-up is shown in Figure 4.
Column elution
The experimental set-up in Figure 4 was also used for column elution experiments. Dow XUS43605 was loaded at a flow rate of 5 BV per hour, a temperature of 60°C, and an initial solution pH of 4. The resulting loadings of metals on- the resin were 21.86 g/ℓ copper, 2.634 g/ℓ nickel, 7 ppm cobalt, 42 ppm iron, and less than 5 ppm zinc, manganese, and aluminium. To achieve homogenously loaded resin, the resin was mixed after having been loaded in separate batches. 10 mℓ of loaded Dow XUS43605 was packed into the glass columns and the metals on the resin were eluted by pumping H2SO4 through the packed resin bed using a peristaltic pump. Samples of the eluate were collected at the bottom of each column.
Analyses
The concentrations of the metals in the synthetic bioleach solutions and all barren solutions were analysed using flame atomic absorption spectroscopy.
Results
Resin screening
The resins listed in Table III were screened based on their suitability to recover copper selectively from the bioleach solution. The separation factors for copper, nickel, and cobalt over iron for the various resins are listed in Table VI. The separation factor for metal A over metal B, (¾, is defined as the ratio of the distribution of metal A between the resin and solution phases to the distribution of metal B, as shown in Equation [1]. D¡ refers to the distribution of metal i, C,r refers to the concentration of metal i on the resin, and Qs refers to the concentration of metal i in solution.

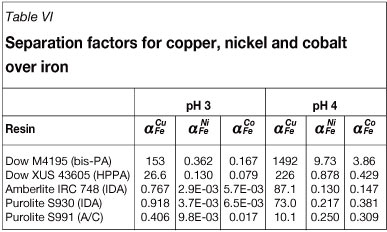
All the resins showed high selectivity towards copper, while the IDA-type resins and A/C-type resin also showed high selectivity towards iron. It is known that difficulties are encountered in the elution of copper from Dow M4195 as the stability constant of the copper-bis-PA complex is high, and conventional acids are thus not able to elute copper. The stability constants of all metals are lower for Dow XUS43605, thus copper could be eluted from this resin using sulphuric acid (Marston and Rodgers 2010).
Although Amberlite IRC748 and Purolite S930 are both IDA-type resins, differences in their selectivity sequences were observed, which were probably caused by differences in synthesis procedures resulting in variations in the structure of the matrix, degree of cross-linking, particle size, density of the functional groups, as well as the proportion of IDA groups. The degree of cross-linking impacts the porosity of a resin and thus also its moisture content, which in turn affects the selectivity of a resin. Dow XUS43605 was selected for subsequent test work based on the results of the screening tests.
Kinetics of adsorption
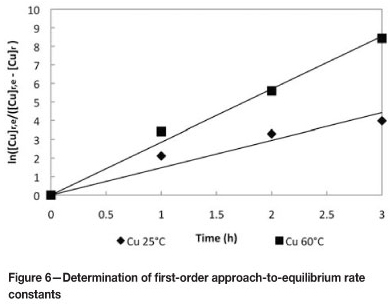
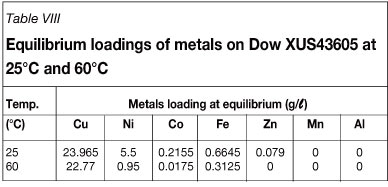
Batch kinetic experiments were conducted to determine the rates of copper loading onto Dow XUS43605 at 25°C and 60°C. The results are shown in Figure 5. A first-order approach-to-equilibrium kinetic model was used to determine the initial rates of copper adsorption, k25°C and k60°C, onto Dow XUS43605. This is illustrated in Figure 6. The first-order rate constants for copper adsorption at 25°C and 60°C are reported in Table VII. It can be seen that the rate of approach to copper adsorption equilibrium increased from 1.47 h-1 to 2.85 h-1 when the temperature was increased from 25°C to 60°C, while the equilibrium loading of copper decreased from 23.97 g/ℓ to 22.77 g/ℓ for this increase in temperature (Table VIII). Although the rate of copper adsorption increased as the temperature was increased, the equilibrium concentration of copper on the resin phase decreased. The increased adsorption rate can be ascribed to the lower viscosity of the leach solution at the higher temperature and increased intra-particle mass transfer, while- the decreased equilibrium loading of copper on the resin could be explained by the exothermic nature of the ion exchange reaction (Sirola 2009).
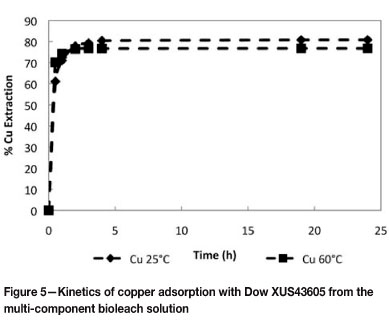
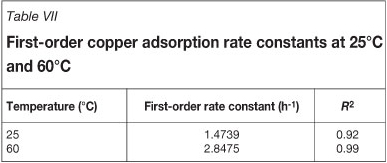
Similar results were obtained by Sirola et al. (2010), who reported that the rate of copper adsorption onto silica-supported 2-(aminomethyl)pyridine increased with increasing temperature in the range of 25°C to 90°C. A discrepancy, however, was noted between the present study and the study by Sirola et al. (2010). In the present study the equilibrium loading of copper was found to decrease with increasing temperature, which is qualitatively what one would expect given the exothermic nature of the ion exchange reaction, while Sirola et al. (2010) reported an increase in equilibrium loading of copper on silica-supported 2-(aminomethyl)pyridine with an increase in temperature.
The initial copper adsorption rates reported in Table VII at 25°C and 60°C were used to calculate the activation energy of copper adsorption with Dow XUS43605 using the Arrhenius equation (Equation [2]):
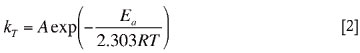
where kT refers to the first-order copper adsorption rate constants at a specific temperature T, Ea represents the activation energy of the copper adsorption reaction, R is the universal gas constant, and A is a pre-exponential constant. The activation energy was calculated to be 24.67 kJ/mol.
Equilibrium adsorption isotherms
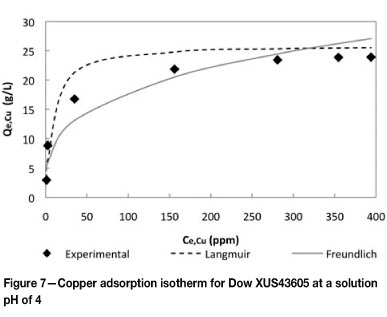
The effect of the resin to solution ratio on the equilibrium concentrations of metals on Dow XUS43605 was investigated by contacting the bioleach solution with the resin at resin-to-solution ratios of 1:5, 1:15, 1:30, 1:50, 1:75, 1:100, and 1:120 at ambient temperature. Single-component Langmuir and Freundlich isotherm models (Equations [3] and [4] respectively) were fitted to the equilibrium data. The results are shown in Figure 7.

In Equations [3] and [4], Cr and Cs refer to the concentration of the metal on the resin and in the solution respectively, while Cr¡ max refers to the maximum concentration of the metal on the resin. The coefficient b is an adsorption equilibrium constant which is a direct measure of the adsorption intensity, and 1/n is a measure of adsorption intensity or surface heterogeneity.
The parameters in the models represented by Equations [3] and [4] are reported in Table IX. It is clear that the Langmuir isotherm model provided a better fit for the experimental data, yielding a fit with an R2 value of 0.96, as opposed to the Freundlich isotherm model, which yielded an R2 value of 0.88.
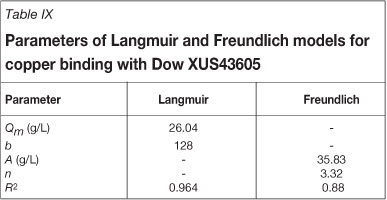
Because these models are generally used to describe the equilibrium of single-component systems, they did not provide fits comparable to such systems as the solution used in this work was more complex in nature. The deviations of the experimentally observed data from the models were especially large in the mid and high copper concentration ranges, and this was believed to be the result of the models not accounting for competition between metals for active sites in a multi-component environment. In these ranges, copper was observed to compete especially with nickel and iron for available sites.
Column adsorption
Effects of operating conditions on metal breakthrough profiles
The effects of flow rate, temperature, pH, and initial metal concentration on metal breakthrough behaviour were studied in dynamic column adsorption experiments. The flow rates tested were 2.5, 5 , and 7.5 BV per hour, the temperatures tested were 25°C and 60°C, and the pH values tested were 3 and 4.
Shown in Figures 8 and 9 are the effects of flow rate on copper and nickel breakthrough profiles at 25°C and 60°C respectively. Because of the relatively fast rate at which copper adsorption with Dow XUS43605 attains equilibrium, the effect of increasing the flow rate from 2.5 BV per hour to 7.5 BV per hour was not extremely significant. The copper loading on the resin decreased from 20.03 g/ℓ to 18.72 g/ℓ when the flow rate increased from 2.5 to 5 BV per hour, and it decreased a further to 16.45 g/ℓ when the flow rate increased from 5 B to 7.5 BV per hour.
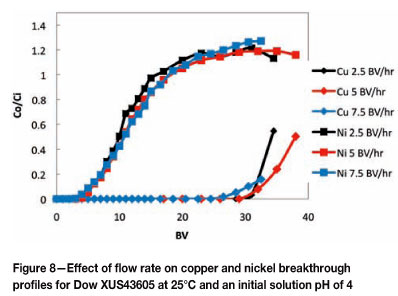
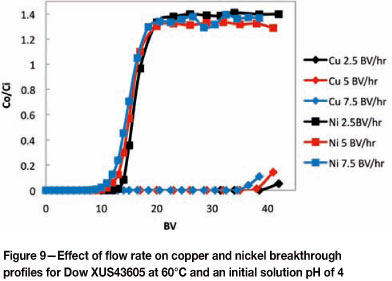
Since the mass transfer kinetics increase as the temperature increases from 25°C to 60°C, breakthrough of copper occurred at a higher BV at 60°C, and thus the copper loading on the resin at copper breakthrough was higher at 60°C. These results illustrate the advantage of operating at an elevated temperature: although the stability constant of copper complexation with Dow XUS43605 decreases with increasing temperature (which corresponds to a lower equilibrium loading of copper on the resin), an increased temperature allows for operation at higher flow rates as higher copper loadings on the resin are achievable at copper breakthrough at 60°C than at 25°C at the same flow rates.
Statistical analysis of metal breakthrough data
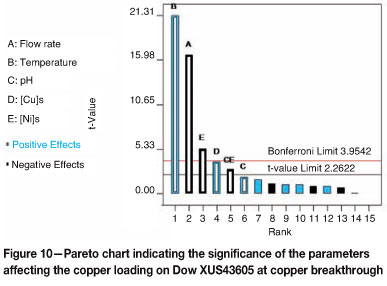
Linear regression was performed on the data for the copper loading on Dow XUS43605 at copper breakthrough to determine the statistical significance of the effects of temperature, flow rate, solution pH, and initial metals concentration on the operating capacity of this resin. Analysis of variance indicated that the effect of temperature was the most significant, followed by the effect of flow rate, and then the effects of the initial nickel and copper concentrations in the solution respectively.
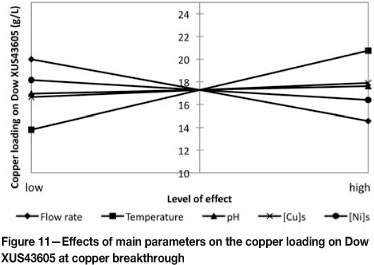
The Pareto chart (Figure 10) furthermore confirmed that temperature had a positive effect, implying that an increase in temperature resulted in an increase of the copper loading on the resin at copper breakthrough. As expected, an increase in the flow rate and initial nickel concentration resulted in a decrease in the copper loading achieved on the resin at copper breakthrough. This is also illustrated in Figure 11, where the copper loadings at copper breakthrough are plotted versus the levels at which the main parameters were tested. The only interaction parameter that was found to be significant was the interaction between pH and the nickel concentration in the solution.
The normalized regression model that was obtained is shown in Equation [5], and it yielded an adjusted R2 value of 0.9811.
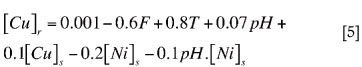
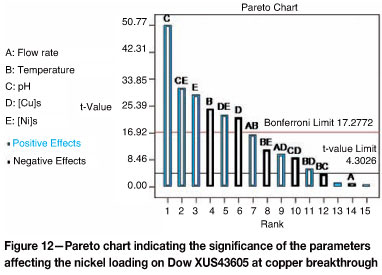
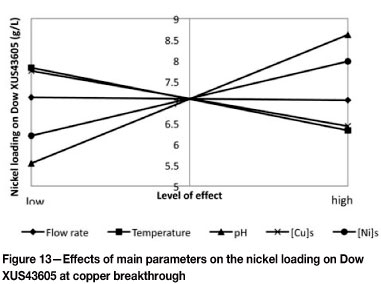
A similar approach was followed to determine the effects of flow rate, temperature, pH, and initial metals concentration on the concentration of co-loaded nickel on Dow XUS43605. Shown in Figure 12 is the Pareto chart indicating the ranking of the parameters and their interactions, as well as whether the impact on the nickel concentration on the resin was positive or negative. As far as the main parameters are concerned, the effect of the initial solution pH was found to be the most significant, followed by the initial nickel concentration, the temperature, and the initial copper concentration, in that order. The initial pH and the initial nickel concentration had positive effects on the nickel loading at copper breakthrough, while an increase in either the temperature or the initial copper concentration resulted in a decrease in the nickel loading at copper breakthrough. This was confirmed by the main parameters plot, shown in Figure 13. Although flow rate did not seem to affect the co-loaded amount of nickel, the interaction effects between the flow rate and temperature and between the flow rate and the initial copper concentration were significant. Other interactions that were found to be statistically significant include those between the temperature and pH with the initial copper and nickel concentrations in solution, as well as the interaction between initial copper and nickel concentration in solution.
Column elution
Dow XUS43605 was loaded in column configuration at a flow rate of 5 BV per hour, an initial solution pH of 4, and a temperature of 60°C. The concentrations of different transition metal ions on the resin were reported in the experimental section.
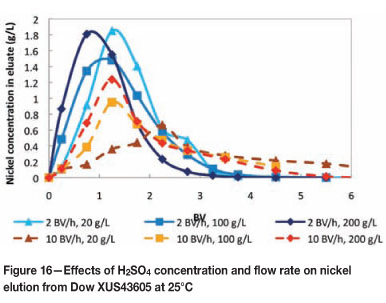
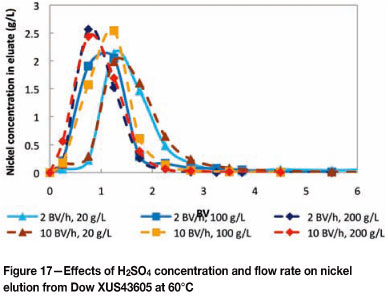
The possibility of performing a split elution of copper and nickel was investigated by eluting Dow XUS43605 with various H2SO4 concentrations (20 g/ℓ, 100 g/ℓ and 200 g/ℓ). The effects of flow rate (2 BV per hour and 10 BV per hour) and temperature (25°C and 60°C) on the elution of metals from Dow XUS43605 were also investigated. The elution of copper from Dow XUS43605 with 20 g/ℓ, 100 g/ℓ, and 200 g/ℓ H2SO4 is shown in Figures 14 and 15, and the elution of nickel is shown in Figures 16 and 17.
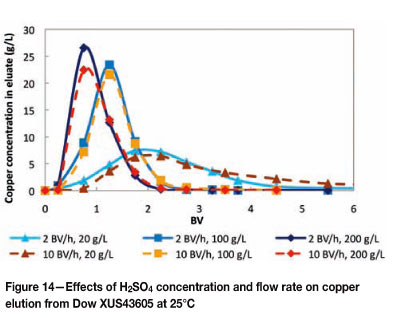
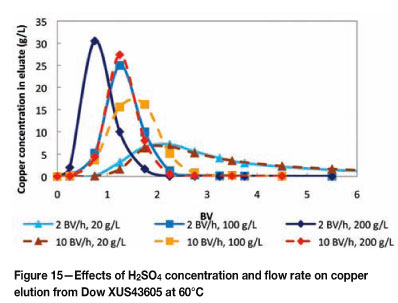
From Figures 14 and 15 it can be seen that the elution of copper was highly dependent on the concentration of H2SO4. On a qualitative basis, the peaks of the elution profiles shifted to higher BVs and the width of the peaks increased as the eluant concentration decreased. The effect of flow rate was less noticeable at 25°C than at 60°C. At 25°C, an increase in the flow rate resulted in slightly lower peaks and wider elution profiles, irrespective of the eluant concentration. The elution profiles at 60°C were slightly slimmer and the peaks were generally higher in relation to the elution profiles at 25°C. Also, an increase in the flow rate shifted the profiles to the right, broadened the profiles, and decreased the peak heights. It was expected that the flow rate would have had a smaller effect at 60°C than at 25°C due to the increased mass transfer kinetics at 60°C relative to 25°C, but the opposite was observed. Therefore further investigation is necessary to clarify this observation. Nevertheless, complete copper elution was still achieved within 2.25 BV of 200 g/ℓ H2SO4 and between 2.5 BV and 3 BV of 100 g/ℓ H2SO4, irrespective of the temperature.
The effect of eluant concentration on nickel elution was less pronounced than in the case of copper elution as a result of the lower stability constant of nickel complexation with Dow XUS43605. Generally, the peaks were wider and the peak heights were lower at 25°C than at 60°C, regardless of the acid concentration. In contrast to the observations made for copper elution, the effect of flow rate on nickel elution was smaller at 60°C than at 25°C. At 25°C, complete nickel elution was achieved with approximately 4 BV of 20 ℓ H2SO4 at a flow rate of 2 BV per hour, while nickel was still not fully eluted after 6 BV of H2SO4 had been passed through the column at a flow rate of 10 BV per hour. The effect of flow rate was not noticeable at 60°C.
These results suggest that a split elution is possible as 90% nickel was eluted with 2 BV of 20 g/ℓ H2SO4 at a flow rate of 10 BV per hour and a temperature of 60°C, while only 35% copper was stripped in the same fraction and under the same conditions. 100% copper elution was achieved with 2 BV of 200 g/ℓ H2SO4 at a flow rate of 10 BV per hour and a temperature of 60°C. It should be noted that, by optimizing the conditions under which adsorption is carried out at, the loading of copper could be maximized and that of nickel could be minimized. The conditions at which the split elution is performed can also be optimized to minimize the amount of copper removed in the nickel-rich eluate fraction (for example by using dilute H2SO4 and a high flow rate). The copper-rich eluate produced in this manner has a copper concentration of 10-15 times higher and a nickel concentration of approxi- mately 4 times lower than in the original bioleach solution, with virtually no other metal impurities, which would allow easier production of copper in downstream processes.
Conclusions
This study investigated the use of Dow XUS43605 for its suitability to selectively recover copper from a multi-component bioleach solution. Screening experiments indicated that the resin has sufficient selectivity towards copper in order to selectively recover it from the bioleach solution, and that the resin has superior iron rejection compared to IDA-type resins.
Batch kinetic experiments indicated that although the equilibrium constant decreases with increasing temperature, the rate of copper adsorption equilibrium attainment increases with increasing temperature. Langmuir and Freundlich isotherms were fitted to batch equilibrium data and the maximum copper capacity of Dow XUS43605 achieved was 26 g/ℓ.
Dynamic column adsorption experiments showed that temperature and flow rate, in this specific order, had the most significant effects on the copper loading on Dow XUS43605 at copper breakthrough. At a flow rate of 5 BV per hour, an increase in temperature from 25°C to 60°C resulted in a 36% increase in the copper loading on the resin at copper breakthrough. In terms of the co-loaded nickel concentration on the resin at copper breakthrough, an increase in the initial solution pH and initial nickel concentration in the solution were found to increase this quantity to a significant extent, while an increase in the temperature affected the nickel concentration negatively.
Finally, it was determined that it is possible to perform a split elution by selecting the appropriate operating conditions. It was found that approximately 90% nickel elution could be achieved with 2 BV of 20 g/ℓ H2SO4 at a flow rate of 10 BV per hour and a temperature of 60°C, while only 35% copper is eluted in the same fraction. A copper-rich eluate could then be obtained by treating the resin with 2 BV of 200 g/ℓ H2SO4. It should be noted that, by optimizing the conditions under which adsorption is carried out at, the loading of copper could be maximized and that of nickel could be minimized, thereby eliminating the need for a split elution to obtain a pure copper product solution.
Acknowledgements
Gratitude is expressed to Lonmin Plc. for financially supporting this project, and also to Dow Water and Process Solutions for supplying Dow XUS43605.
References
Dow Chemical Company. 2011. Ion exchange resins for chemical processing. Dow Water and Process Solutions Manual. [ Links ]
Grinstead, R.R. 1984. Selective absorption of copper, nickel, cobalt and other transition metal ions from sulfuric acid solutions with the chelating ion exchange resin XFS 4195. Hydrometallurgy, vol. 12, no. 3. pp. 387-400. [ Links ]
Habashi, F. 1990. Textbook of Hydrometallurgy. Métallurgie Extractive Quebec, Quebec, Canada. [ Links ]
HO, E. and QUAN, C. 2007. Iron(II) oxidation by SO2/air for use in uranium leaching. Hydrometallurgy, vol. 1, no. 85. pp. 183-192. [ Links ]
Irving, H. and Williams, R. 1953. The stability of transition metal complexes. Journal of the Chemical Society. pp. 192-3210. [ Links ]
MARSTON, C. and RODGERS, M. 2010. Cobalt recovery from copper solvent extraction raffinate with ion exchange. Presented at SME Annual Meeting, Phoenix, USA, 28 February - 3 March 2010. [ Links ]
MENDES, F.D. and MARTINS, A.H. 2005. Selective nickel and cobalt uptake from pressure sulfuric acid leach solutions using column resin sorption. International Journal of Mineral Processing, vol. 77, no. 1. pp. 53-63. [ Links ]
Mendes, F.D. and Martins, A.H. 2004. Selective sorption of nickel and cobalt from sulphate solutions using chelating resins. International Journal of Mineral Processing, vol. 1, no. 74. pp. 359-371. [ Links ]
Monhemius, J. 1981. Hydrometallurgical processing of complex materials. Chemical Industry (London), vol. 20. pp. 410-420. [ Links ]
Mouton, M., Van Deventer, J., and Vaarno, J. 2007. Oxidative precipitation of Fe and Mn by Air/SO2. The Fourth South African Conference on Base Metals. The Southern African Institute of Mining and Metallurgy. [ Links ]
Mwase, J. 2009. Hydrometallurgical extraction of platinum group metals from a low grade ore concentrate. MSc Thesis, University of Cape Town. [ Links ]
Mwase, J., Petersen, J., and Eksteen, J.J. 2012. A conceptual flow sheet for heap leaching of platinum group metals (PGMs) from a low-grade ore concentrate. Hydrometallurgy, vol. 111-112. pp. 129-135. [ Links ]
NICOL, M.J. 2003. Ion exchange and adsorption in hydrometallurgy. Internal Course Notes. Department of Chemical Engineering, University of Cape Town. [ Links ]
Pearson, R. 1963. Hard and soft acids and bases. Journal of the American Chemical Society, vol. 85. pp. 3533-3539. [ Links ]
Rosato, L., Harris, G.B., and Stanley, R.W. 1984. Separation of nickel from cobalt in sulphate medium by ion exchange. Hydrometallurgy, vol. 13, no. 1. pp. 33-44. [ Links ]
Sirola, K. 2009. Chelating adsorbents in the purification of hydrometallurgical solutions. PhD thesis, Acta Universitatis: Lappeenranta University of Technology. [ Links ]
Zainol, Z. and Nicol, M.J. 2010. Comparitive study of chelating ion exchange resins for the recovery of nickel and cobalt from laterite leach tailings. Hydrometallurgy, vol. 96, no. 4. pp. 283-287. [ Links ]
Zhang, W. and Muir, D. 2010. Oxidation of Fe(II) in a synthetic nickel laterite liquor with SO2/air. Minerals Engineering, vol. 1, no. 23. pp. 40-44. [ Links ]
Paper received Feb. 2013
Revised paper received Apr. 2013
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN2225-6253.














