Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 n.4 Johannesburg Apr. 2013
PAPERS
Alpha case formation mechanism in Ti-6Al-4V alloy investment castings using YFSZ shell moulds
by A.M. BauristheneI; K. MutomboII; W.E. StumpfI
IDepartment of Materials Science and Metallurgical Engineering, University of Pretoria, Pretoria
IIDepartment of Materials Science and Manufacturing, Council for Scientific and Industrial Research, Pretoria
SYNOPSIS
Ti-64, which accounts for more than 50% of the worldwide titanium tonnage, has found commercial importance in industries requiring components with high specific strength and resistance to corrosion. Investment casting is the preferred production method due to the difficult machinability of the alloy. This study was aimed at investigating the mechanism and the extent of alpha case formation on Ti-64 components cast using the investment casting method with YFSZ (yttria fully-stabilized zirconia) shell moulds after vacuum induction melting. The extent of the reaction between the mould hot face and the molten metal has been studied by varying parameters such as soaking temperature and mould hot face composition, and examining their effects on the reaction with the mould. An increase in the soaking temperature had an effect on the alpha case, both in appearance and hardness, but had no effect on contamination levels by carbon, oxygen, and nitrogen. The depth of alpha case increased with soaking temperature, increasing from 35 µm to 161 µm with an increase in temperature from 1200°C to 1400°C. The micro-hardness profiles provided insight into the effect of the alpha case on the mechanical properties of the Ti-64 alloy by displaying hardness values of 1000 HV0.1 and above, but could not be solely utilized to determine the alpha case penetration depth due to microstructural differences in the unaffected Ti-64, in particular the martensitic microstructure that formed with a fast cooling rate from a higher temperature. Levels of expected contaminants such as Zr, Y, O, and C were low. The addition of the colloidal zirconia binder affected the interfacial reactions. YFSZ proved to be a thermodynamically stable refractory material, with the alpha case possibly forming as a result of segregation.
Keywords: Ti-6Al-4V, alpha case, investment casting, contamination, YFSZ shell moulds.
Introduction
The titanium base alloy Ti-6Al-4V (Ti-64), which accounts for more than half of the titanium metal tonnage in the world (Boyer et al., 1994), has found commercial importance for a number of reasons, including superior mechanical properties in both wrought and cast forms, such as a higher strength-to-density ratio and improved environmental resistance compared to other materials. The commercial aerospace and military industries make the most use of Ti-64, taking advantage of the superior mechanical properties and low density of the alloy. However, the alloy is finding applications in the biomedical and chemical processing industries due to the corrosion resistance and biocompatibility of the base metal titanium (Dimcic et al., 2005). These industries require that the components are intricately designed, and the difficult machinability and low ductility of titanium alloys negates the use of conventional forming processes. Therefore the components are preferably cast using investment casting or permanent mould casting. The increased cost of titanium alloys, due to the complexity of the extraction processes as well as losses during secondary processing operations, and induced by the formation of an alpha case layer during casting (Mutombo and Rossouw, 2011) increases the difficulty of post-cast processing such as mechanical surface cleaning. This has therefore restricted their use in the automotive industry, although the high price and environmental impacts of energy consumption are encouraging weight reductions in transportation vehicles, which can potentially be offered by Ti-64 (Ucok et al., 2007).
The formation mechanism of alpha case on Ti-64 investment castings using yttria fully-stabilized zirconia (YFSZ) shell moulds was investigated by studying the effects of controllable reaction variables such as soaking temperature and mould hot face composition, with the expectation that reaction temperature will have the greatest impact.
Ti-6Al-4V
Substitutional alloying elements
Alloying elements have varying effects on the properties and phase compositions of titanium. Some alloying elements, such as alpha stabilizers, enlarge the alpha phase region on a phase diagram with aluminium, oxygen and nitrogen (Boyer et al., 1994; Donachie, 2000) as typical examples. On the other hand, beta-stabilizing elements include vanadium and zirconium, and were particularly important for this study (Donachie 2000; Lin and Lin, 1999). In the context of this study it is important to note that Ti-64 contains a mixture of alpha and beta stabilizers in the form of aluminium and vanadium respectively, and is therefore an alpha-beta titanium alloy at ambient temperature (Donachie, 2000).
Aluminium increases the strength by solid solution strengthening, but a content of less than 7 wt% is desirable because the alloy becomes susceptible to environmental embrittlement at higher aluminium contents. Aluminium has an extra benefit of limiting the surface oxidation of titanium by forming a dense and stable oxide of its own that restricts the diffusion of oxygen through the heterogeneous oxides of titanium and aluminium (Lütjering and Williams, 2007). Sung and Kim (2005) also suggested that an intermetallic titanium aluminide phase is formed that adds to the alpha case. The alpha-stabilizing aluminium addition is balanced by the addition of vanadium as a beta-stabilizing element, which is rejected from the alpha phase due to the low solubility of vanadium in this phase, so that a mixture of alpha and beta can be obtained in the final alloy. The beta-stabilizing elements can aid in solid solution strengthening and retard the formation of alpha so that beta is retained (Donachie, 2000) as well as strengthening the overall alloy by retarding grain coarsening and thereby refining the microstructure.
Interstitial impurities
Oxygen and nitrogen react with the surface layer of titanium, resulting in a structure that is composed predominantly of alpha phase, since both interstitials are alpha stabilizers. Oxygen is soluble in titanium up to 3.5 wt% at 673K and has a significant hardening effect (Saha et al., 1999). The oxygen-enriched surface forms an alpha case and allows oxygen to diffuse through it to reach and react with the underlying base metal. This alpha case, along with the oxygen-rich layer in the base metal, is detrimental to Ti-64 because of the brittle nature of the alpha phase, and can result in surface cracks under tension (Lütjering and Williams, 2007). The alpha case, therefore, needs to be removed before the component is put into service because it is susceptible to crack initiation and propagation and is approximately twice as hard as the base metal (Mutombo and Rossouw, 2011). The alpha case can be seen in Figure 1 as the darker material on the left of the sample. The removal can be carried out by machining or by aggressive chemical milling. The latter method is preferred because of the abrasiveness of the alpha case to machine tools, and chemical milling is also able to remove the alpha case in intricately designed castings. The alpha case can extend to depths of up to 2 mm under severe casting conditions (Mutombo and Rossouw, 2011) and up to 0.2 mm in heat treatments.

Yttria fully-stabilized zirconia
Mutombo and Rossouw (2011) suggested that the monoclinic phase of zirconia is the most reactive and therefore should be kept at minimum levels when zirconia-based shell moulds are used for titanium alloy casting. Additions of yttria to zirconia in the weight range of 4 wt% to 8 wt% produce a partially stabilized zirconia, and additions in excess of 8 wt% produce a fully stabilized zirconia. The face-coat is composed mainly of zirconia and yttria, but Mutombo and Rossouw (2011) published results indicating the presence of impurity oxides such as HfO2, Al2O3, and CaO, amongst others.
Casting thermodynamics
An example of the thermodynamic consideration of the metal-mould interface reaction applicable to this study is taken from Lin and Lin (1999), where it is proven that the interfacial reactions involving molten titanium and solid zirconia at 1727°C are not thermodynamically feasible, and it can be inferred that titanium cannot reduce zirconia. Lin and Lin found, however, that ZrO2 had been transformed to ZrO2-x and a gaseous phase had evolved. The presence of this oxygen-deficient zirconia and titanium sub-oxides such as Ti2O and Ti3O indicated that zirconia was partially reduced by titanium during solidification, implying that the metal-mould interface reaction between titanium alloys and a zirconia hot face cannot be predicted based solely on thermodynamic considerations.
Experimental procedure
Small cylinders with a longitudinal cavity were machined from as-received imported Ti-64 ingots with threaded plugs to seal the cavity. All the samples and plugs were handled with gloves to avoid surface contamination from the skin. The cavities of the samples were filled to volume capacity with one of the following powders:
► 80% passing 44 µm of 8% YFSZ
► 80% passing 44 µm YFSZ mixed with colloidal zirconia binder.
The plug was screwed securely into place before the exterior surfaces were thickly painted with yttria paint in order to protect the sample from atmospheric contamination in the furnace. Once dry, the samples were placed in the furnace in an air atmosphere and reacted at temperatures of 1000°C, 1200°C, and 1400°C for one hour. Upon completion the remaining powder was removed from the cavities; the samples were sectioned, mounted, polished, and etched with Kroll's etchant in preparation for the metallographic analyses: optical microscopy, Vickers micro-indentation hardness testing, scanning electron microscopy (SEM), and energy-dispersive spectroscopy (EDS).
Results and discussion
Fine yttria fully-stabilised zirconia
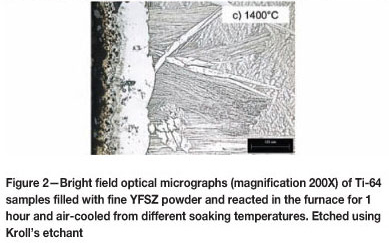
In the bright field optical micrographs of Figure 2, the alpha phase is the light coloured phase and the beta phase is the grey coloured phase.
In Figure 2(a), close to the reaction interface, some grains display a full alpha structure as opposed to the typical Widmanstátten microstructure within other grains. This is an indication of an alpha case forming, and using spatial calibration software these alpha grains could be found 190 µm deep into the base metal. Within the larger grains of Figure 2(b) and (c), a needle-like martensitic microstructure exists which had formed at the higher temperature from which the sample was cooled and the fast cooling rate enforced by air cooling. A solid alpha phase is present at the reaction interface of the specimen in Figures 2(b) and (c), and no other precipitation of alpha phase is present in the area. Finger-like protrusions of alpha phase, extending up to 620 µm into the bulk metal, can be noticed in Figure 2(c). The solid alpha case is estimated at 35 µm deep and 161 µm deep in Figures 2(b) and (c) respectively. A porous type structure can be noticed at the immediate reaction interface of Figure 2(c), the effects of which will be studied more closely in a later section. The presence of the alpha case increases the hardness to well above the as-received hardness, as shown in Figure 3.
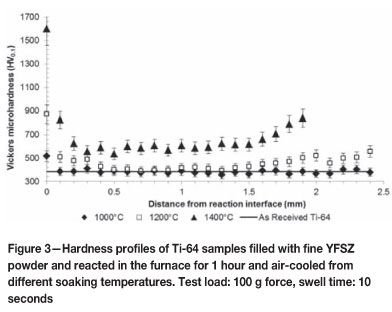
Note that the sample exposed to a temperature of 1000°C (Figure 2a) displays markedly lower hardness values than those of the samples exposed to soaking temperatures of 1200°C and 1400°C across the distance range of reaction interface to bulk metal. At roughly 200 µm depth, similar to the thickness estimated by Figure 2(a), the hardness of this sample drops to the hardness of the as-received Ti-64 and oscillates around this value of 390 HVcn, indicating the end of the alpha case. The solid alpha case in the sample exposed to a temperature of 1200°C more than doubles the near-surface hardness of the as-received alloy, and according to the profile the alpha case should end at roughly 700 µπι. This is not the situation when compared to Figure 2(b). The martensitic microstructure increases the hardness of the base metal to above the as-received hardness across the entire distance range until there is a marked increase in hardness at 1.4 mm, where the alpha phase precipitating from the outside surface in towards the reaction interface influences the hardness values. This suggests that the yttria paint does not serve as suitable protection against atmospheric contamination within the furnace.
The extreme hardness at the immediate interface of the sample exposed to a soaking temperature of 1400°C can be attributed to the porous structure noticed in Figure 2(c), which was studied further by EDS. The second data point in this particular set, which lies in the alpha case, is therefore a more reliable value for the alpha case hardness. The martensitic microstructure affects the hardness by increasing the bulk hardness to about 600 HV0.1 and therefore the as-received hardness is not reached. By estimating the bulk hardness, the alpha case depth can be estimated to be 300 µm. External surface contaminants also affect the hardness, evident by the increase in hardness after 1.5 mm, which leads to the same conclusion as presented above with regard to the yttria paint.
SEM-EDS analysis was used to generate the maps in Figure 4. The alpha phase is the darker phase in the secondary electron image (SEI). As can be seen from the maps, there is no contamination from yttrium, zirconium, or oxygen. The only contamination is in the form of carbon. Noticeable features are the areas of depleted vanadium associated with the alpha case. A high concentration of vanadium immediately adjacent to the alpha case is also noticeable. There is an aluminium-deficient area along with an increased aluminium and titanium concentration immediately adjacent to the high-vanadium area. This is important to note, because of the phase preference of both these elements when alloyed with titanium: aluminium is more soluble in the alpha phase whereas vanadium is more soluble in the beta phase. Within the aluminium-deficient area there is an increased concentration of carbon and an even distribution of titanium. This suggests interstitial contamination of the alloy with carbon.
Fine yttria fully-stabilized zirconia plus colloidal zirconia binder
In the micrographs in Figure 5(a) and (b), a solid alpha case is present with areas of seemingly porous microstructure at the immediate reaction interface. Large grains with martensitic microstructures are present in both specimens, as well as alpha-phase grain boundaries. Colonies of alpha-phase needles, which are not present in Figure 5(b), are present near the alpha case in Figure 5(a). The alpha case depths can be estimated as 23 µm and 35 µm for Figures 5(a) and (b) respectively.
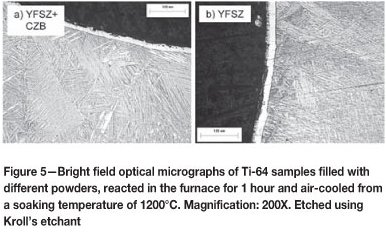
Considering the hardness profiles in Figure 6, a difference in hardness of roughly 125 HV0.1 at the immediate reaction interface is noted. Thereafter the profiles are similar in shape with some noticeable features: the bulk hardness for both specimens is greater than the as-received hardness of 390 HV0.1 as a result of the martensitic microstructure described in Figures 5(a) and (b). The specimen reacted with YFSZ plus CZB, correlating to Figure 5(a), displays a higher bulk hardness than the specimen reacted with only YFSZ. This appears to be due to an increased amount of alpha phase present in the bulk microstructure, evident from the colonies of alpha phase present in Figure 5(a).
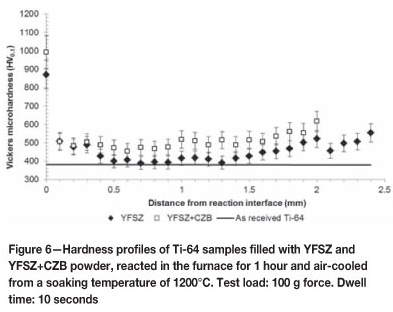
The second, third, and fourth data points from the respective data sets display equal hardnesses at the same distance from the reaction interface up to 300 µm deep. After this distance, the hardness of the specimen reacted with YFSZ decreases and approaches the as-received hardness, whereas the hardness of the specimen reacted with YFSZ plus CZB remains at this hardness of roughly 500 HV0.1. At a distance of 1.5 mm from the reaction interface the hardness begins to increase again due to the effects of the surface contamination picked up from the atmosphere within the furnace, proving that although the yttria paint hindered the atmospheric contamination of the surface, it was not completely successful in preventing it.
EDS analysis was performed on the specimens, generating the maps in Figure 7. Localized contamination in the form of Ca and C, is noticed in the alpha case immediately adjacent to the reaction interface, whereas an area depleted in vanadium coincides with the same alpha case. The presence of Ca as an impurity suggests that the YFSZ plus CZB was contaminated.
Conclusions
An increase in the soaking temperature had an effect on both the appearance and hardness the alpha case, but had no effect on contamination levels. The depth of alpha case penetration has shown the expected trend with an increase in soaking temperature, increasing from 35 µm to 161 µm with an increase in temperature from 1200°C to 1400°C. The micro-hardness profiles gave insight into the effects of the alpha case on the mechanical properties of the Ti-64 alloy, displaying interfacial hardness values in excess of 1000 HV0.1, but the profiles cannot be utilized alone to determine the alpha case penetration depth, due to microstructural differences in the base metal engendered during cooling, which influence the hardness readings. Levels of expected contaminants such as Zr, Y, O, and C were low. The EDS results do, however, give insight into whether there was contamination from other elements such as Ca and C during the experiment.
The addition of the colloidal zirconia binder affected the interfacial reactions, as is evident from the micrographs and microhardness profiles. YFSZ proves to be a thermodynamically stable refractory material, as it is not reduced by the Ti and does not contaminate the alloy. The yttria paint, however, did not fully prevent the pick-up of atmospheric surface contamination, but it did hinder the oxidation of titanium at high temperatures.
The temperatures used for the experiments are well above the alpha-beta transition temperature for Ti-64, and therefore a phase change occurs upon cooling from high temperatures. Vanadium is more soluble in the beta phase than in the alpha phase, and from the EDS maps it is clear that the alpha case is depleted in vanadium. A possible cause for this is the segregation of less-soluble elements from the area of the specimen that undergoes the beta-alpha transformation before the bulk metal reaches the transition temperature. This possibly induces differing cooling rates in the reaction interface and in the bulk metal. A quicker cooling rate at the reaction interface may result from the refractory nature of the mould powder used during the experiments.
Acknowledgements
The authors would like to thank the Department of Materials Science and Manufacturing at the CSIR for supplying the materials used in the study and allowing the use of the laboratory facilities. The authors would also like to thank the University of Pretoria and the CSIR for their permission to publish this paper.
References
Collings, E.W. 1994. Technical Note 3: Casting. Materials Properties Handbook - Titanium Alloys. Boyer, R., Welsch, G., and Collings, E.W. (eds.). ASM International. Metals Park, Ohio. pp. 1079-1082. [ Links ]
Dimcic, B., Bobic, I., Zec, S., and Jovanovic, M.T. 2005. Appearance of a hard layer ("α-case") on the surface of two different titanium-based alloys. 2nd International Conference: Deformation Processing and Structure of Materials, Belgrade, Serbia and Montenegro, 26-28 May 2005. Institute of Nuclear Sciences. pp. 207-211. [ Links ]
Donachie, M.J. 2000. Titanium - A Technical Guide. 2nd edn. ASM International, Metals Park, Ohio. [ Links ]
Lin, K/-F. and Lin, C.-C. 1999. Interfacial reactions between Ti-6Al-4V alloy and zirconia mould during casting. Journal of Material Science, vol. 34. pp. 5899-5906. [ Links ]
Lütjering, G. and Williams, J.C. 2007. Titanium. 2nd edn. Springer-Verlag, New York. [ Links ]
Mutombo, K. and Rossouw, P. 2011. Effect of pickling solution on the surface morphology of Ti6Al4V alloy investment cast. CSIR, Pretoria, South Africa. [ Links ]
Saha, R.L., Nandy, T.K., Misra, R.D.K., and Jacob, K.T. 1989. Evaluation of the reactivity of titanium with mould materials during casting. Bulletin of Material Science, vol. 12, no. 5. pp. 481-493. [ Links ]
Sung, S.-Y. and Kim, Y.-J. 2005. Alpha-case formation mechanism on titanium investment castings. Materials Science and Engineering A, vol. 405. pp. 173-177. [ Links ]
Ucok, I., Dong, H., Klug, K.L., Kramer, L.S., Gungor, M.N., and Tack, W.T. 2007. Microstructure and pmechanical Properties of Ti-6Al-4V investment castings. Innovations in Titanium Technology. Gungor, M.N., Imam, M.A., and Froes, F.H. (eds.). John Wiley & Sons. Orlando, Florida. pp. 189-198. [ Links ]
Paper received Dec. 2012
Revised paper received Dec. 2012.
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN2225-6253
Paper written on project work carried out in partial fulfilment of B. Eng (Metallurgy)