Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 n.2 Johannesburg Feb. 2013
Combining bainite and martensite in steel microstructures for light weight applications
M.J. SantofimiaI; S.M.C. van BohemenII; J. SietsmaIII
IDelft University of Technology, Delft, the Netherlands and Materials innovation institute M2i, Delft, the Netherlands
IITata Steel RD&T, IJmuiden, the Netherlands
IIIDelft University of Technology, Delft, the Netherlands
SYNOPSIS
Multiphase microstructures in steel have been intensively studied in the past years, but combining non-equilibrium phases still offers a great potential for further development of these steel grades. Thus, improved combinations of mechanical properties can be obtained with microstructures formed by bainite or martensite, in combination with retained austenite. In particular, microstructures on the basis of carbon-depleted martensite and retained austenite can be produced by the very promising production process named quenching and partitioning (Q&P). Originally, the Q&P process aimed to avoid the formation of bainite during the heat treatment. However, the process does provide the possibility for (carbide-free) bainite formation during the partitioning step, i.e. in the presence of martensite. This article evaluates this approach, considering that the formation of bainite from austenite is strongly influenced by the preceding formation of martensite. Although the accelerating effect of the presence of martensite during bainite formation has been observed, it is not yet fully understood, and experimental and theoretical studies are being performed in order to come to a more effective exploitation of these processes for the formation of multiphase microstructures.
Keywords: quenching and partitioning, bainite, martensite.
Introduction
Nowadays, the market offers advanced high-strength steels (AHSS) with improved combinations of strength and ductility with respect to conventional steels (Figure 1). Most of the AHSS are based on multiphase microstructures. Thus, for example, dual phase (DP) steels have microstructures containing martensite and ferrite. Further developments led to the design of steels containing more complex microstructures, for instance consisting of combinations of ferrite, bainite, martensite, and retained austenite.
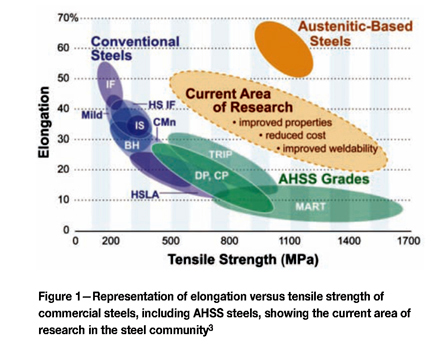
The interest in having retained austenite in the microstructure is due to the ability of this metastable phase to transform into martensite during the application of mechanical loading, which significantly contributes to the strengthening of the material. This phenomenon is called the transformation-induced plasticity (TRIP) effect, and steels having such multiphase microstructures are named TRIP steels. Martensitic (MART in Figure 1) steels are based on fully martensitic microstructures, leading to high levels of strength, but these materials perform poorly on elongation.
New applications in the automotive and energy sectors are demanding steels with improved combinations of mechanical properties. These demands defined a new research area for the design of new steels, as indicated in Figure 1. New strategies to fulfil these demands are based on the design of more attractive steels with ultra-fine grained microstructures, containing combinations of strong phases such as martensite and bainite. The presence of retained austenite in these microstructures is also desirable in order to make use of the TRIP effect1 2.
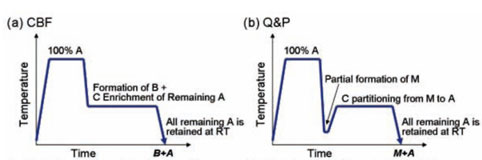
Heat treatments for the development of carbide-free bainite (CFB) steels consist of full austenitization followed by an isothermal step at a temperature in the bainite range4 (Figure 2a). The formation of bainite occurs by the nucleation and displacive growth of bainitic ferrite sub-units until they reach a certain size. In the process, carbon diffuses from the carbon-supersaturated sub-unit to the surrounding austenite5. By alloying the steel with elements such as silicon or aluminium, carbide precipitation in the austenite is inhibited, and thus the austenite becomes carbon enriched as the transformation progresses.
In the case of the quenching and partitioning (Q&P) process6, the route consists of a first quench, from a full or partially austenitic microstructure, to induce a partial martensitic transformation, followed by an isothermal treatment to accomplish the carbon partitioning from martensite to austenite in the absence of carbide precipitation (Figure 2b). Carbon-enriched austenite can thus remain metastable at room temperature7.
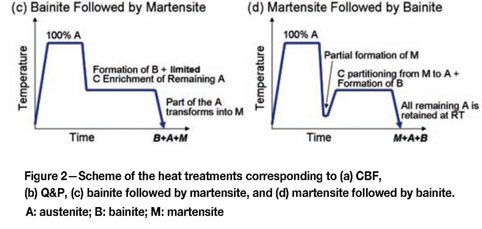
In this work, the possibility of combining both processing concepts to create microstructures containing combinations of martensite, bainite, and retained austenite is evaluated. First, the inconveniences of creating these microstructures via bainite formation followed by martensite formation (Figure 2c) will be discussed. We then continue with a description of the approach to create such multiphase microstructures via the formation of martensite followed by bainite formation (Figure 2d), in which the carbon enrichment of the austenite occurs by a combination of the mechanisms used in CFB and Q&P steels. The article ends with a description of modelling approach for the microstructure development of this new processing route.
Bainite formation followed by martensite
Microstructures consisting of bainite, martensite, and retained austenite are relatively easy to form through a processing route starting with full austenitization, followed by an isothermal step at a temperature in the bainite range, followed by a further quench (Figure 2c). If the material is quenched to room temperature before the austenite reaches sufficient carbon enrichment to be retained at room temperature, part of the austenite transforms into martensite. These regions of 'fresh' (i.e. untempered) martensite contain a carbon concentration higher than the nominal carbon concentration of the steel, and have detrimental effects on the mechanical properties of the steels8, especially the edge ductility (hole expansion). In fact, also in the design of CBF steels, it is very important to adequately select chemical compositions and heat treatments that will maximize the formation of bainite and the carbon enrichment of austenite in the microstructure such that the formation of high-carbon martensite islands is avoided. For these reasons, this is not an interesting approach for the creation of microstructures formed by martensite, bainite, and retained austenite.
Martensite formation followed by bainite
Proposed heat treatment
The key steps of the Q&P process are the partial formation of martensite during the quenching step and the carbon partitioning from the supersaturated martensite to the surrounding austenite.
If austenite becomes sufficiently carbon-enriched, it remains fully retained at room temperature. The remaining constituent in the microstructure is carbon-depleted martensite, which has beneficial effects on the mechanical properties. In fact, the martensite is tempered during the partitioning step, but without the formation of carbides. As in the case of CFB microstructures, islands of high-carbon martensite are to be avoided by an adequate design of compositions and heat treatments.
The process of carbon partitioning from martensite to austenite typically takes place at intermediate temperatures, at which the formation of bainite is in principle possible. However, previous research has shown that if materials and treatments are properly selected, the formation of bainite can be sufficiently delayed so that the carbon diffusion from martensite to austenite occurs without any interference9. In these steels, the carbon enrichment of the austenite occurs faster via carbon partitioning from the martensite than via formation of bainite. Therefore, it was generally accepted that a proper control in Q&P treatments requires the inhibition of the formation of bainite.
However, it is interesting to evaluate the consequences of the promotion of the bainite formation during the partitioning step in these steels. In this framework, it is important to keep in mind that the formation of bainite from austenite is strongly influenced by preceding formation of martensite, as was already qualitatively observed by Steven and Haynes in 195610. Thus, the kinetics of bainite formation occurs faster in a microstructure partially transformed into martensite than in a full austenite microstructure, for the same steel and transformation temperature. This phenomenon is highly relevant for the Q&P process.
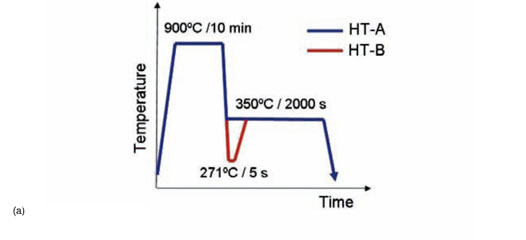
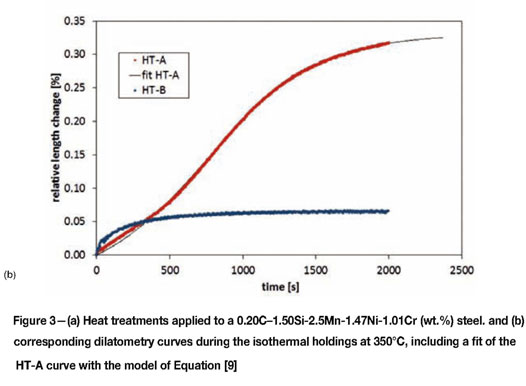
The phenomenon is displayed in Figure 3. Figure 3a shows two heat treatments applied to a 0.20C-1.50Si-2.5Mn-1.47Ni-1.01Cr (wt.%) steel. Heat treatment HT-A consists of a full austenitization followed by a rapid cooling to 350°C, which is a temperature in the bainitic range, at which the material is held for 2000 s. HT-B corresponds to full austenitization, rapid cooling to 271°C, during which a volume fraction of martensite equal to 0.63 is formed (according to the fit shown in Equation [9]), and an isothermal treatment at 350°C for 2000 s. The heat treatments have been applied in a dilatometer. Figure 3b shows the relative change in length measured in both cases during the isothermal treatments at 350°C for 2000 s. The dilatometry curves show expansions, corresponding to the formation of bainite, as explained in Santofimia et al.9. The volume fractions of bainite formed during the isothermal holding of HT-A is clearly higher than in HT-B. However, the kinetics of bainite formation is clearly faster in the case of HT-B. Since the kinetics of diffusionless bainite formation is governed by nucleation, the reason for this is probably the presence of additional nucleation sites before the isothermal holding in HT-B. These additional nucleation sites were created during the partial quench to 271°C in the form or martensite/austenite interphases.
These observations open a possibility for an alternative approach to the Q&P process, in which the carbon enrichment of the austenite is reached by a combination of carbon partitioning from the martensite and the formation of carbide-free bainite (Figure 2d). The advantages of such a strategy to create microstructures comprising carbon-depleted martensite, carbide-free bainite, and retained austenite are manifold. First, the time required for the carbon enrichment of the austenite is significantly reduced. In addition, since the carbon enrichment of the austenite occurs by two different processes, grains of retained austenite in the final microstructure are expected to have a wider range of carbon concentrations than in the case of steels processed by conventional Q&P and CFB routes, and consequently offer more possibilities for microstructural design. Since the mechanical stability of the retained austenite upon deformation depends on its carbon concentration, a variety of carbon concentrations of retained austenite would lead to a more gradual TRIP response of the austenite upon deformation, and a consequently increased strain hardening.
Modelling the microstructure development
In the following sections some theoretical concepts will be considered that are relevant to modelling the microstructure development associated with the proposed heat treatment.
Partial transformation of austenite into martensite
The proposed heat treatment in Figure 2d starts with an austenitization and subsequent quench, during which the microstructure is partially transformed to martensite. The volume fraction of martensite (fª) formed as a function of undercooling below the martensite start temperature (Ms) can be described by the Koistinen and Marburger (KM) equation":
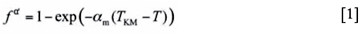
where am is a rate parameter that depends on the composition12 and TKM is a theoretical temperature indicating the start of the exponential relation between temperature and martensite fraction, and which can be somewhat lower (typically 5°C to 20°C) than the temperature at which the first martensite forms, the martensite start temperature Ms.
Predictions using the KM equation of the fraction of martensite at a certain arrest temperature below the Ms temperature are not only dependent on a proper estimate of TKM, but are also strongly influenced by the rate parameter am controlling the temperature dependence of martensite formation. Van Bohemen and Sietsma12 observed that am is composition-dependent and that carbon has a dominant effect on am. It has been found that for low-carbon steels (0.1-0.2 wt.% C), the value of am is approximately 0.022 K-1, whereas it decreases with increasing carbon content. Thus, for steels with approximately 1 wt.% carbon, the rate parameter am is close to 0.011 K-1, which is the value originally proposed by Koistinen and Marburger in 1959. For a certain composition range, empirical equations for the composition dependence of am were proposed in recent studies12,13.
Modelling carbon partitioning
The first approach to model the kinetics of carbon partitioning from martensite to austenite was presented by Speer et al.14-17, who proposed that the carbon flux from the martensite to the austenite is governed by the so-called 'constrained carbon equilibrium' (CCE). The CCE considers that iron and substitutional atoms are essentially immobile at temperatures at which the carbon partitioning takes place, so only carbon equilibrates its chemical potential, whereas the martensite-austenite interface is assumed immobile or stationary.
Experimental observations questioning the immobility of the martensite-austenite interface18,19 led Speer et al.20 to relax the CCE, including the possibility of interface migration. In this new approach, the difference in iron potential between the ferrite and the austenite creates a driving force for iron to change its structure from one phase to the other, which is accomplished via migration of the existing interface, assuming that nucleation of new crystals does not occur.
Under these considerations, the present authors21,22 developed a model in which it was assumed that the chemical potential of carbon in martensite and austenite is the same at the interface, and that the interface migrates when a free-energy difference occurs. In the model, martensite is considered to have a body-centered cubic (bcc) structure supersaturated in carbon, whereas austenite is the usual face-centered cubic (fcc) phase. The model considers the same chemical potential of carbon in bcc and in fcc at the bcc-fcc interface because of the high atomic mobility of interstitial carbon. This condition can be expressed in terms of carbon concentration by21
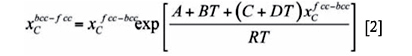
where 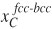 and
and 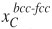 are the carbon concentrations in fcc and bcc at the interfaces, respectively, T is the temperature, R is the universal gas constant and A, B, C, and D are constants that depend on the chemical composition of both phases and can be determined, for a particular chemical composition, using thermodynamic databases.
are the carbon concentrations in fcc and bcc at the interfaces, respectively, T is the temperature, R is the universal gas constant and A, B, C, and D are constants that depend on the chemical composition of both phases and can be determined, for a particular chemical composition, using thermodynamic databases.
The motion of interfaces in a microstructure is a result of the repositioning of atoms from lattice positions in one grain to projected lattice positions in a neighbouring grain. At a given temperature, the equilibrium concentrations of carbon in cc,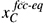 , and bcc,
, and bcc,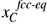 , are given by the metastable equilibrium phase diagram, for which carbide formation is excluded. If the carbon concentrations at the interface are different from the metastable equilibrium values, the phases will experience a driving pressure, ΔG, for a phase transformation towards the equilibrium phase composition. This local driving pressure is experienced at the interface and results in an interface velocity, v, which is proportional to the driving pressure according to:
, are given by the metastable equilibrium phase diagram, for which carbide formation is excluded. If the carbon concentrations at the interface are different from the metastable equilibrium values, the phases will experience a driving pressure, ΔG, for a phase transformation towards the equilibrium phase composition. This local driving pressure is experienced at the interface and results in an interface velocity, v, which is proportional to the driving pressure according to:

with M the interface mobility. In this model, the driving pressure is provided by the difference in the iron chemical potentials, and is considered proportional to the difference between 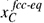 and
and 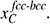 The driving pressure can be positive or negative, depending on the relative difference between the equilibrium carbon content of the austenite and the actual carbon concentration in austenite at the interface.
The driving pressure can be positive or negative, depending on the relative difference between the equilibrium carbon content of the austenite and the actual carbon concentration in austenite at the interface.
The relationship between 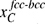 and the interface migration behaviour, according to the present model, is schematically represented in Figure 4. If the interface is enriched in carbon relative to equilibrium, the chemical potential of iron is higher in martensite than in austenite and the driving pressure for the movement of the interface promotes interface migration from the austenite to the martensite (Figure 4a), whereas the interface would be promoted to move in the opposite direction if the interface is depleted in carbon relative to equilibrium (Figure 4b).
and the interface migration behaviour, according to the present model, is schematically represented in Figure 4. If the interface is enriched in carbon relative to equilibrium, the chemical potential of iron is higher in martensite than in austenite and the driving pressure for the movement of the interface promotes interface migration from the austenite to the martensite (Figure 4a), whereas the interface would be promoted to move in the opposite direction if the interface is depleted in carbon relative to equilibrium (Figure 4b).
The interface mobility, which is temperature-dependent, can be expressed by an Arrhenius equation according to:
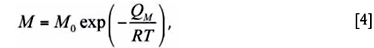
where QM is the activation energy for iron atom motion at the interface and M0 a pre-exponential factor.
Further comparison between calculations with this model and experimental data showed that the extent of martensite-austenite interface migration during the process of carbon partitioning from martensite to austenite is small, suggesting that the mobility of these interfaces is low9
Modelling the formation of bainite
There are several models for the description of the kinetics of bainite formation in the literature. The model presented by Van Bohemen and Sietsma23 is based on a displacive mechanism for bainite growth. Nucleation of bainite sub-units is assumed to take place at austenite grain boundaries and continue through autocatalytic nucleation. In this model, the rate of bainite nucleation at a temperature T is given by:
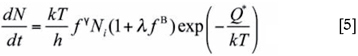
where N is the nucleation density, k is the Boltzmann constant, h is the Planck constant, fy is the volume fraction of austenite, Ni is the initial density of potential nucleation sites, λ is the autocatalytic constant, fb is the bainite fraction, Q* is the activation energy. The volume fraction fy of untransformed austenite (which equals 1-fB if only austenite and bainite are present) accounts for the decrease in the number of potential nucleation sites with increasing volume fraction of bainite fB with time t. The autocatalytic nucleation is incorporated by the factor (1 + λfb).
The initial nucleation-site density Ni is considered to increase with the undercooling below the bainite start temperature Th as:

where Γ = d(ΔGm)/dT is the increase in the driving force with the temperature decrease. In accordance with martensite nucleation24 it can be assumed that φ = α / VbΓ, which leads to:

In this equation, α is an experimental parameter that has not yet been investigated for bainite, but only for martensite, yielding values between 0.01 and 0.03 K-1, dependent on chemical composition. It reflects the density of potential nucleation sites in relation to the undercooling.
Since the growth of bainite is very fast and the average volume of bainite sub-units is constant over the extent of the transformation, the change in fraction can be calculated directly from the nucleation rate according to:

By substituting Equation [5] in Equation [8], it follows that isothermal bainite formation is governed by the differential equation:
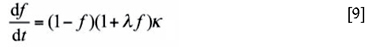
with κ a rate parameter, given23 . The solution of this equation provides the kinetics of the isothermal formation of 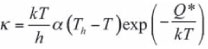 bainite. It is shown in Figure 3b that the isothermal bainite formation in heat treatment A can be adequately modelled with this equation, with λ = 7.2 and κ = 3.2 x 10-4 s-1. This model has been also applied to model the formation of bainite during cooling, leading to satisfactory agreement with experimental results25.
bainite. It is shown in Figure 3b that the isothermal bainite formation in heat treatment A can be adequately modelled with this equation, with λ = 7.2 and κ = 3.2 x 10-4 s-1. This model has been also applied to model the formation of bainite during cooling, leading to satisfactory agreement with experimental results25.
Modelling the overlap of processes
The formation of martensite prior to the partitioning step may affect the bainite formation kinetics in two ways. First, the presence of martensite reduces the volume fraction of austenite available for the formation of bainite, which is evidenced by the smaller length change observed in Figure 3b. Second, martensite/austenite boundaries seem to act as nucleation sites for bainite, leading to an acceleration of the overall bainite transformation kinetics. This second effect leads to a higher value for the parameter α in Equation [7], and is subsequently displayed by the rate parameter for the dilatation curve of heat treatment B in Figure 3b being 80% higher than for the bainite formation without the presence of martensite (curve HT-A in Figure 3b). Both effects are thus taken into account in Equation [9] for the overall kinetics of bainite formation, although the present experimental results do not allow for a determination of the λ-parameter for bainite formation in the presence of martensite.
With respect to the overlap between the process of carbon partitioning and the formation of bainite, both mechanisms lead to an enhanced carbon enrichment of the austenite. The change in carbon of the austenite due to the formation of bainite affects the balance between carbon in martensite and in austenite expressed by Equation [2], and therefore, the rate at which austenite will 'attract' carbon from the martensite. Similarly, the increase of the carbon concentration in the austenite through the carbon partitioning from martensite would accelerate the rate at which the austenite reaches the maximum carbon content at which the formation of bainite is stopped.
Qualitatively, it is clear that the coupling of both processes would lead to an overall acceleration of the process of carbon enrichment of the austenite. Previous sections provide guidelines to follow for the creation of quantitative models describing the microstructure development during the proposed route and present preliminary experimental results on the phenomena. Further work is needed to fully understand and control these mechanisms.
Conclusion
In this work, a new thermal route for the creation of microstructures containing carbon-depleted martensite, bainite, and retained austenite is proposed, based on concepts from the Q&P and CBF processes. This new thermal route makes positive use of the accelerating effect that martensite has on the kinetics of bainite formation. Guidelines for the creation of models for the microstructure development during the application of this thermal route are suggested.
Acknowledgements
This research was carried out under the project number M41.09246 in the framework of the Research Program of the Materials Innovation Institute M2i (www.m2i.nl). The support of TATA Steel RD&T for this project is acknowledged.
References
1. De Moor, E., Gibbs, P.J., Speer, J.G., and Matlock, D.K. Strategies for third-generation advanced high strength steel development. AIST Transactions, vol. 7, 2010. pp. 133--144. [ Links ]
2. Matlock, D.K. and Speer, J.G. Third generation of AHSS: microstructure design concepts. Microstructure and Texture in Steels. Part II. 2009. pp. 185-205. [ Links ]
3. World Steel Organisation. http://www.worldsteel.org [ Links ]
4. Caballero, F.G., Santofimia, M.J., Garcia-Mateo, C., Chao, J., and Garcia de Andres, C. Theoretical design and advanced microstructure in super high strength steels. Materials and Design, vol. 30, 2009. pp. 2077-2083. [ Links ]
5. Bhadeshia, H.K.D.H. Bainitie in Steels. The Institute of Materials, London, 2001. [ Links ]
6. Speer, J.G., Rizzo, F.C., Matlock, D.K., and Edmonds, D.V. The quenching and partitioning process: background and recent progress. Materials Research, vol. 8, 2005. pp. 417-423. [ Links ]
7. Edmonds, D.V., He, K., Miller, M.K., Rizzo, F.C., Clarke, A., Matlock, D.K., and Speer, J.G. Microstructural features of quenching and partitioning: a new martensitic steel treatment. Materials Science Forum, vol. 539-543, 2007. pp. 4819-4825. [ Links ]
8. Bhadeshia, H.K.D.H. and Edmonds, D.V. Bainite in silicon steels. New composition-property approach. Part 1. Metal Science, vol. 17, 1983. pp. 411-419. [ Links ]
9. Santofimia, M.J., Zhao, L., Petrov, R., Kwakernaak, C., Sloof, W.G., and Sietsma, J. Microstructural changes occurring during the quenching and partitioning process in a newly designed low-carbon steel. Acta Materialia, vol. 59, 2011. pp. 6059-6068. [ Links ]
10. Steven, W. and Haynes, A.G. The temperature of formation of martensite and bainite in low-alloy steels. Journal of the Iron and Steel Institute, vol. 183, 1956. pp. 349-359. [ Links ]
11. Koistinen, D.P. and Marburger, R.E. A general equation prescribing the extent of the austenite-martensite transformation in pure iron-carbon alloys and plain carbon steels. Acta Metallurgica, vol. 7, 1959. pp. 59-60. [ Links ]
12. Van Bohemen, S.M.C. and Sietsma, J. Effect of composition on kinetics of athermal martensite formation in plain carbon steels. Materials Science and Technology, vol. 25, 2009. pp. 1009-1012. [ Links ]
13. Van Bohemen, S.M.C. Bainite and martensite start temperature calculated with exponential carbon dependence. Materials Science and Technology, vol. 28, 2012. pp. 487--495. [ Links ]
14. Speer, J.G., Matlock, D.K., De Cooman, B.C., and Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Materialia, vol. 51, 2003. pp. 2611-2622. [ Links ]
15. Speer, J.G., Matlock, D.K., De Cooman, B.C., and Schroth, J.G. Comments on 'On the definitions of paraequilibrium and orthoequilibrium'. Scripta Materialia, vol. 52, 2005. pp. 83-85. [ Links ]
16. Hillert, M. and Ågren, J. On the definitions of paraequilibrium and orthoequilibrium. Scripta Materialia, vol. 50, 2004. pp. 697-699. [ Links ]
17. Hillert, M. and Ågren, J. Reply to comments on 'On the definition of paraequilibrium and orthoequilibrium'. Scripta Materialia, vol. 52, 2005. pp. 87-88. [ Links ]
18. Zhong, N., Wang, X., Rong, Y., and Wang, L. Interface migration between martensite and austenite during the quenching and partitioning process. Journal of Materials Science and Technology, vol. 22, 2006. pp. 751-754. [ Links ]
19. Kim, S.J., Kim, H.S., and De Cooman, B.C. Dilatometric study of the quench and partitioning (Q&P) process. Materials Science and Technology (MS&T), Detroit, Michigan, 2007. pp. 73-83. [ Links ]
20. Speer, J.G., Hackenberg, R.E., De Cooman, B.C., and Matlock, D.K. Influence of interface migration during annealing of martensite/austenite mixtures. Philosophical Magazine Letters, vol. 87, 2007. pp. 379-382. [ Links ]
21. Santofimia, M.J., Zhao, L., and Sietsma, J. Model for the interaction between interface migration and carbon diffusion during annealing of martensite-austenite microstructures in steels. Scripta Materialia, vol. 59. 2008.pp.159-162. [ Links ]
22. Santofimia, M.J., Speer, J.G., Clarke, A.J., Zhao, L., and Sietsma, J. Influence of interface mobility on the evolution of austenite-martensite grain assemblies during annealing. Acta Materialia, vol. 57, 2009. pp. 4548-4557. [ Links ]
23. Van Bohemen, S.M.C. and Sietsma, J. Modelling of isothermal bainite formation based on the nucleation kinetics. International Journal of Material Research, vol. 7, 2008. pp. 739-747. [ Links ]
24. Magee, C.L. The nucleation of martensite. Phase Transformations., ASM International, Metals Park, OH, 1970. pp. 115-156. [ Links ]
25. Van Bohemen, S.M.C. and Sietsma, J. The kinetics of bainite and martensite formation in steels during cooling. Materials Science and Engineering A, vol. 527, 2012. pp. 6672-6676. [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN2225-6253.
This paper was first presented at the, Ferrous and Base Metals Development Network Conference 2012, 15-17 October 2012, Mount Grace Country House and Spa, Magaliesburg, South Africa..