Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 no.2 Johannesburg Fev. 2013
Evaluation of oxine-type ligand coordination to zirconium (IV)
M. Steyn; H.G. Visser; A. Roodt
Department of Chemistry, University of the Free State
SYNOPSIS
[Zr(C9H6NO)4]-(HCON(CH3)2)-(H2O), where (C9H6NO) = 8-hydroxy quinoline (oxH), was synthesized and characterized. This tetrakis-coordinated zirconium complex crystallized in the triclinic crystal system (PT, Z=2) along with water and N,N'-dimethylformamide (DMF) solvate in the asymmetric unit. The metal atom is surrounded by four N,O-donating bidentate ox-ligands that are arranged around the metal centre to give a square antiprismatic coordination polyhedron with a small distortion towards a dodeca-hedral geometry. Crystal packing is stabilized by intermolecular interactions of adjacent oxine ring systems in neighbouring molecules, as well as hydrogen bonding of the aqua and DMF solvate molecules, linking the molecular entities into a supramol-ecular three-dimensional network.
Keywords: zirconium, tetrakis coordination, quinolinato.
Introduction
Zirconium metal complexes have received moderate amounts of interest from research groups worldwide. The general focus of research concerning zirconium as coordinated metal revolves around structural defining studies with particular reference to coordination geometnes1-5. Other research reports focus heavily on the development of new complexes for industrial applications in catalysis, with particular interest in polymerization catalytic processes6-10. Although many literature sources discuss in detail the similarities and differences between zirconium and their aimed investigative comparative metal(s), few ever focus in such detail on the specific associated zirconium formation/coordination chemistry processes.
This study forms part of ongoing research initiatives investigating the coordination behaviour of O,O'- and N,O-bidentate ligands with zirconium(IV) and hafnium(IV) for possible separation of these two metals from base ore sources.
Experimental
General considerations
All reagents used for the synthesis and characterization were of analytical grade and were purchased from Sigma-Aldrich, South Africa. Reagents were used as received, without further purification.
The reaction solvent used throughout, N,N'-dimethyl formamide (DMF), was dried by passing through alumina. Infrared spectra were recorded on a Bruker Tensor 27 Standard System spectrophotometer with a laser range of 4000-370 cm-1, coupled to a computer. All 1H NMR spectra were obtained in C6D6 on a Bruker 300 MHz nuclear magnetic resonance spectrometer.
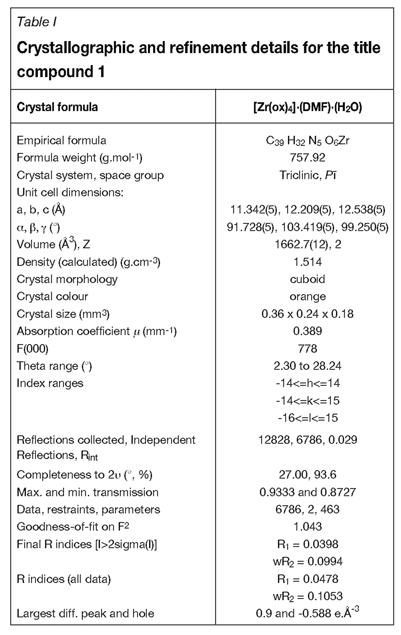
Crystallography
The X-ray intensity data was collected on a Bruker X8 ApexII 4K Kappa CCD area detector diffractometer, equipped with a graphite monochromator and MoKa fine-focus sealed tube (λ = 0.71069 Ǻ, T = 100(2) K) operated at 2.0 kW (50 kV, 40 mA). The initial unit cell determinations and data collections were done by the SMART software package11. The collected frames were integrated using a narrow-frame integration algorithm and reduced with the Bruker SAINT-Plus and XPREP software packages12 respectively. Analysis of the data showed no significant decay during the data collection. Data was corrected for absorption effects using the multi-scan technique SADABS13 and the structure was solved by the direct methods package SIR9714 and refined using the WinGX15 software incorporating SHELXL16.
The final anisotropic full-matrix least-squares refinement was done on F2. The methine, methylene, and aromatic protons were placed in geometrically idealized positions (C-H = 0.93 -0.98 Ǻ) and constrained to ride on their parent atoms with Uiso(H) = 1.2Ueq(C). Non-hydrogen atoms were refined with anisotropic displacement parameters. The graphics were obtained with the DIAMOND17 program with 50 per cent probability ellipsoids for all non-hydrogen atoms.
Synthesis of [Zr(ox)4]-(DMF)-(H2O)
ZrCl4 (0.1003 g, 0.430 mmol) and oxH (0.1413 g, 0.863 mmol) were each separately dissolved in 10 ml DMF. These reagent solutions were heated to ca. 40.0 °C before the ligand solution was added dropwiseto the metal solution while stirring. The final reaction solution was left to stir at the elevated temperature for 30 minutes before removal of the reaction vessel to a fume hood and allowing to stand for crystallization under slow evaporation over several days. (Yield: mass ca. 51%); IR (ATR): vCO = 1572 cm-1; UV/Vis:
λmax= 382 nm, ε = 157 cm-1.M-1; 1H NMR (Benzene-d6): δ = 6.70 (d, 1H, J = 6 Hz), 7.29 (dd, 2H, J = 7.8 Hz, 6 Hz), 7.36 (t, 2H, J = 7.8 Hz), 8.13 (d, 1H, J = 7.2 Hz).
Results and discussion
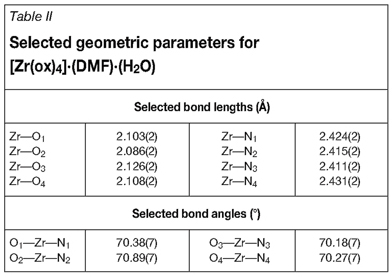
Crystal data and details of the data collection and refinement are shown in Table I and selected geometric parameters are given in Table II. The numbering scheme for this DMF-solvated complex in its unit cell is given in Figure 1. Hydrogen atoms and/or solvent molecules are omitted in some molecular presentations for clarity.
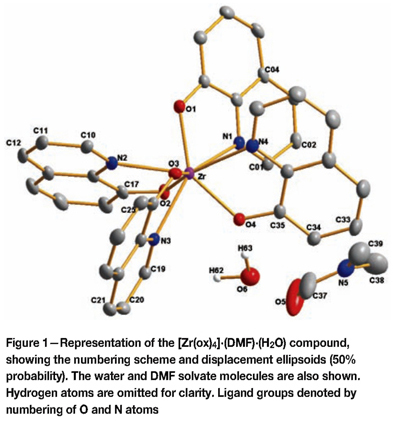
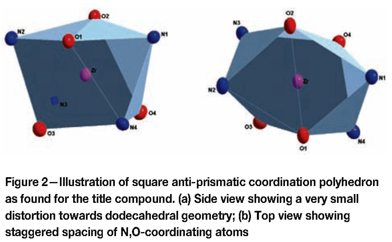
The title compound is composed of an eight-coordinated zirconium metal centre, in which the four N,O-donating bidentate ox-ligands are arranged around the metal centre to give a square antiprismatic coordination polyhedron with a small distortion towards a dodecahedral geometry (see Figure 2). The ox-ligands are arranged in an approximately equally spaced placement in the three-dimensional space around the metal centre, with alternating placement of the coordinating O and N atoms from the ligand, i.e. approaching an inversion-centred coordination geometry.
The bite angles of the bidentate ligands range from 70.11(1) to 70.84(1)°, with Zr-O bond distances averaging 2.106(2) Ǻ and Zr-N bond distances averaging 2.420(2) Ǻ. The opposite facing ox-ligand groups, however, do not form part of the same plane. These planes, created by each ligand group respectively, lie at an angle to each other with an average of 42.52° with its opposite facing ligand group. Each unit cell contains two molecular units consisting of a Zr-complex, a DMF solvent molecule, and a water molecule (See Figure 3).
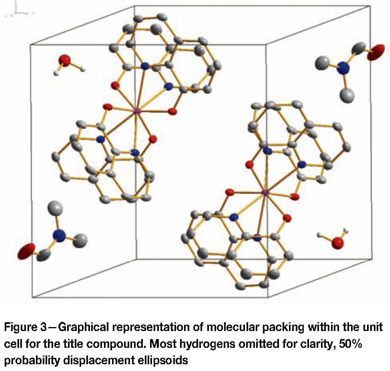
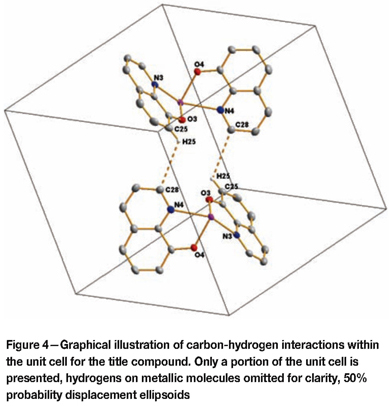
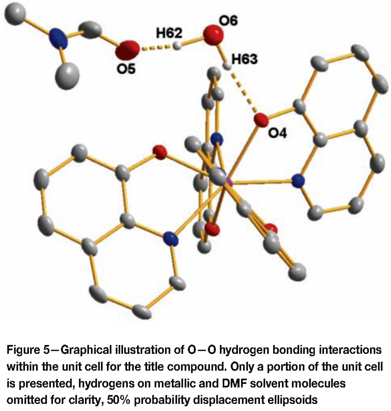
The metallic portion of the molecular units is positioned at the centre of the unit cell and the solvent molecules span the corners of the unit cell. Within the unit cell, interactions between the metallic molecular groups are observed on the outermost carbons for the 3rd and 4th ligand groups on mirrored counterparts (Figure 4) within the unit cell: C25- H25 to C28(sym. code: 1-x, 1-y, 1-z) - 2.214 Ǻ, 123.82°. Further interactions are observed (see Figure 5) between the O from the solvent water molecule to the O on the DMF carbonyl from a neighbouring unit cell - O6-H62 to O5 (sym. code: 2-x, -y, 2-z) - 1.653 Ǻ, 163.61°.
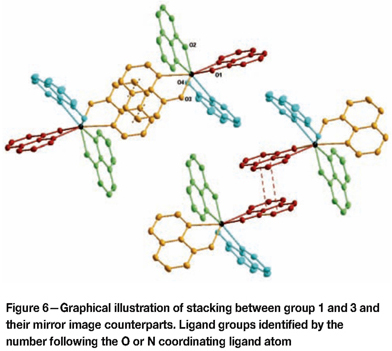
π-π stacking is observed between ligand groups 1 and 3 with their mirror images from neighbouring unit cells (see Figure 6) as reported in Table III. These ligand groups span the outermost edge of the unit cell (as seen in Figure 3) and as such, every unit cell shows two of these sets of stacked ligand group partnerships. This π-π stacking show a complete overlay of quinoline ligand groups for each pair.
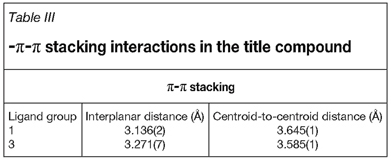
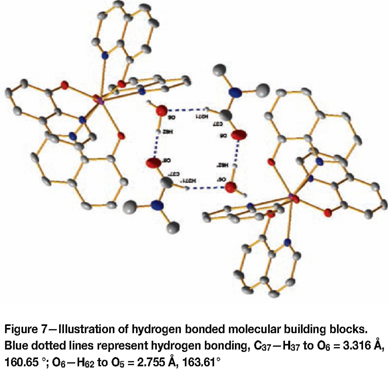
The most noteworthy aspect visible in the crystal packing is the evidence of molecular building block groups structured around a backbone of the solvent molecules (see Figure 7). Hydrogen bonding is observed between solvent molecules of neighbouring unit cells. These units constructed from two molecular units, consisting of an organometallic portion and a solvation portion, form the building blocks for the entire structure.
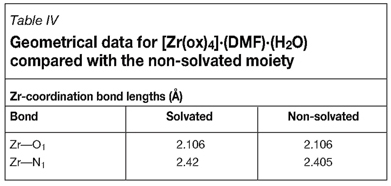
Lewis et al.18 published the first [Zr(ox)4] structure in 1974. The only structural information given in this publication is the zirconium to coordinating ligand atom distances, which were compared to the identical distances for other metal complexes. As can be seen in Table IV, the distances they reported are near identical to the solvated structure discussed in this section. This indicates that solvent contributions to zirconium complex packing are negligible, if not non-existent.
Conclusion
The new zirconium crystal structure ([Zr(ox)4]-(DMF)-(H2O)) has been successfully characterized. Comparisons were drawn with similar structures from the literature and the structure from this study was found to conform well known theories of zirconium - bidentate ligand coordination crystallography. The zirconium structure discussed here also conforms to what is generally accepted as the blueprint of zirconium - bidentate type ligand coordination geometry, namely the square antiprismatic coordination mode as well as general structural characteristics such as bond lengths and bonding bite angles. Very few structures of this type (ox) of bidentate ligand organometallic structures are known in the literature though, leaving large scope for future studies on the same ligands and derivatives.
Acknowledgement
Financial assistance from the Advanced Metals Initiative (AMI) of the Department of Science and Technology (DST) of South Africa, through the New Metals Development Network (NMDN) coordinated by the South African Nuclear Energy Corporation Limited (NECSA), is gratefully acknowledged.
References
1. Silverton, J.V. and Hoard, J.L. Article title. Inorganic Chemistry, vol. 2, 1963. pp. 243-249. [ Links ]
2. Pinnavaia, T.J. and Fay, R.C. Article title. Inorganic Chemistry, vol. 7, 1968. pp. 502-508. [ Links ]
3. Chun, H.K., Steffen, W.L., and Fay, R.C. Article title. Inorganic Chemistry, vol. 18, 1979. pp. 2458-2465. [ Links ]
4. CLEGG, W. Article title. ActaCrystallographica, vol. C43, 1987. pp. 789-793. [ Links ]
5. Calderazzo, F., Englert, U., Maichle-Mõssmer, C., Marchetti, F., Pampaloni, G., Petroni, D., Pinzino, C., Stràhle, J., and Tripepi, G. Article title. Inorganica Chimica Acta, vol. 270, 1998. pp. 177-188. [ Links ]
6. Rasika Dias, H.V., Jin, W., and Wang, Z. Article title. Inorganic Chemistry, vol. 35, 1996. pp. 6074-. [ Links ]
7. Scott, M.J. and Lippard, S.J. Article title. Inorganica Chimica Acta, vol. 263, 1997. pp. 287-299. [ Links ]
8. Rahim, M., Taylor, N.J., Xin, S., and Collins, S. Article title. Organometallics, vol. 17, 1998. pp. 1315-. [ Links ]
9. Vollmerhaus, R., Rahim, M., Tomaszewski, R., Xin, S., Taylor , N.J., and Collins, S. Article title. Organometallics, vol. 19, 2000. pp. 2161-2169. [ Links ]
10. Kim, J., Hwang, J.W., Kim, Y., Lee, M.H., Han, Y., and Do, Y. Article title. Journal of Organometallic Chemistry, vol. 620, 2001.pp. 1-7. [ Links ]
11. Bruker AXS Inc. Bruker SMART-NT Version 5.050. Area-Detector Software Package. Madison, WI, USA.1998. [ Links ]
12. Bruker SAINT-PLUS Version 6.02 (including XPREP), Area-Detector Integration Software, Madison, WI, USA, 1999. [ Links ]
13. Bruker AXS Inc. Bruker SADABS Version 2004/1.Bruker AXS Inc. Area Detector Absorption Correction Software. Madison, WI, USA, 1998. [ Links ]
14. Altomare, A., Burla, M.C., Camalli, M., Cascarano, G.L., Giacovazzo, C., Guagliardi, A., Moliterni, A.G.G., Polidori, G., and Spagna, R. Article title. Journal of Applied Crystallography, vol. 32, 1999. pp. 115-119. [ Links ]
15. Farrugia, L.J. Article title. Journal of Applied Crystallography, vol. 32,1999. pp. 837-838. [ Links ]
16. Sheldrick, G.M. SHELXL97. Program for crystal structure refinement. University of Gottingen, Germany, 1997. [ Links ]
17. Brandenburg, K. and Putz, H. Diamond, Release 3.0e.Crystal Impact GbR, Bonn, Germany, 2006. [ Links ]
18. Lewis, D.F. and Fay, R.C. Article title. J. Chem. Soc. Chem. Comm. pp. 1046-1047. [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN2225-6253.
This paper was first presented at the, Ferrous and Base Metals Development Network Conference 2012, 15-17 October 2012, Mount Grace Country House and Spa, Magaliesburg, South Africa..














