Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 n.2 Johannesburg Feb. 2013
A comparative assessment of Ti-47.5 at.%Al cathodically modified by precious metal addition
LA. MwambaI, II; L.A. CornishII; E. van der LingenI
IAdvanced Materials Division, Mintek and School of Chemical and Metallurgical Engineering and DST/NRF Centre of Excellence in Strong Materials, University of the Witwatersrand
IISchool of Chemical and Metallurgical Engineering and DST/NRF Centre of Excellence in Strong Materials, University of the Witwatersrand
SYNOPSIS
Plain and alloyed titanium aluminides of composition Ti-47.5 at.% Al were prepared by melting commercial-purity titanium and aluminium with additions of 1 at.% precious metal. The as-cast alloys were subjected to potentiodynamic scans in 5, 15, and 25 wt% HCl aqueous solutions at room temperature and compared for their abilities to spontaneously passivate.
Addition of precious metals resulted in a general improvement of corrosion resistance by increasing the open circuit potential to more noble values. Addition of 1 at.% gold or silver to titanium aluminide did not significantly increase the corrosion potential (ecorr) above that of the plain titanium aluminide alloy, indicating that gold and silver are not sufficient cathodic modifiers to improve the corrosion resistance of titanium aluminide in all the solutions tested. However, platinum, palladium, and iridium additions shifted the corrosion potentials to the position of the passive region of plain TiAl for all solution concentrations. This indicated that TiAl alloyed with these platinum group metals would passivate spontaneously by cathodic modification. TiAl alloyed with palladium performed the best in 5 wt% HCl solution with the most positive corrosion potential. In 15 wt% HCl solution, alloys with platinum exhibited the most positive corrosion potentials, while alloying with iridium or palladium revealed more negative corrosion potentials. Thus, palladium alloying led to the best cathodic modification of titanium aluminide in low-concentration HCl solution, whereas platinum and iridium alloying additions resulted in the best cathodic modification of titanium aluminide in high-concentration HCl solutions.
Keywords: cathodic modification, precious metals, titanium, corrosion.
Introduction
Titanium aluminides are alloys with high potential for elevated temperature applications in turbine components due to their strength at high temperatures, light weight, and good oxidation properties1-5. Much work has been dedicated to the investigation of the high-temperature oxidation properties of titanium aluminides, resulting in much available data. Although much work was done on the corrosion behaviour of pure titanium and titanium-based alloys, very limited information is available on the room-temperature or near room-temperature aqueous corrosion properties of titanium aluminides. This is due to the fact that most investigations conducted to date on the titanium aluminide alloys were aimed at high-temperature performance, and therefore, high-temperature properties such as oxidation and corrosion resistances were investigated. Gamma titanium aluminide, originally designed for high-temperature applications, appears to have excellent potential for bone repair and replacement, with the biological response expected to be similar to other titanium-base biomaterials6. These observations from early tests have triggered recent research activities on γ-TiAl as implants for the human body7. In the process, recent years have seen a growing interest in assessing the corrosion properties of γ-TiAl in aqueous media simulating human body fluids, which justify the need to generate a database on the corrosion properties of titanium aluminide in aqueous solutions8.
Some catastrophic structure failures have occurred in various alloys due to lack of room-temperature corrosion resistance resulting in stress corrosion cracking9-10. Investigations of many alloy systems were triggered by corrosion-induced failure of structures in daily life. For instance, crevice corrosion studies of titanium and its alloys in sodium chloride in chemical industries where this metal and its alloys are extensively used. In most aqueous environments, titanium and titanium alloys show good corrosion resistance, due to the stable oxide films that form spontaneously on the surface. However, in chloride and/or fluoride solutions, titanium alloys are susceptible to attack, showing pitting and crevice corrosion11. Alloying elements, including nickel and molybdenum, have been added to titanium and titanium alloys to improve the corrosion resistance to pitting12-13. Precious metals were added to titanium to increase the corrosion resistance in non-oxidizing acids14-15.
Addition of precious metals as alloying elements to improve corrosion resistance was not limited to titanium and its alloys. Precious metals were also used to increase the corrosion resistance of other alloys, such as stainless steel16-17. Their use for corrosion resistance improvement originated in 1911, when Monnartz reported that the rapid corrosion of iron-chromium alloys in certain acids could be suppressed by winding a platinum wire around the corrosion test specimen or by adding platinum as an alloying element to steel18. Stainless steel and nickel-based alloys were alloyed with other elements in order to improve their corrosion resistance19-21.
Several methods for controlling or improving corrosion resistance of metals and alloys have been reported22. Corrosion resistance can be controlled and/or improved by cathodic modification23-24. For some metals and their alloys, such as aluminium, modification of anodic layers has been also used to improve corrosion resistance25-27. Beneficial alloying elements mostly used in cathodic modification include platinum group metals, and nickel and/or molybdenum13,28. When present in stainless steel, molybdenum led to better corrosion resistance by cathodic modification than molybdenum-free stainless steel20.
Platinum group metal (PGM), nickel, or molybdenum additions facilitate cathodic depolarization by providing sites of low hydrogen overvoltage, which shifts alloy potential in the positive direction where oxide film passivation is possible24,29. a comprehensive description of the cathodic modification mechanism was given by Potgieter15,23. Relatively small concentrations of certain precious metals, of the order of 0.1 wt%, are sufficient to expand significantly the corrosion resistance of titanium in reducing acid media.
For alloys used in critical engine components, such as turbine blades and turbocharger casings, fuel combustion results in the formation of by-products, which form corrosive solutions once in contact with a moisturizing environment, leading to pitting and crevice corrosion30-31 in addition to the hot corrosion usually observed in gas turbine components32-33. Alloying plays an important role in the corrosion resistance of materials in such environments, as the microstructural components of the alloy have different responses if subjected individually to the same environment. It is also difficult to predict the response of the alloy to the corrosive medium, as the presence of new phases, compounds, and structures can lead to pitting and/or galvanic corrosion. In this work, titanium aluminide alloys of α2+γ and γ compositions with 1 at.% precious metal additions were prepared and assessed for pitting corrosion resistance. The precious metals were assessed for their ability to cathodically modify the titanium aluminide alloys.
Experimental procedure
Production of samples
TiAl alloys were prepared by melting Grade 2 titanium and CP aluminium in a button arc furnace under an argon atmosphere. The target composition was Ti-47.5 at.% Al (α2 + γ) for the plain TiAl alloys. Plain TiAl and TiAl alloyed with precious metals (PMs), e.g. silver, gold, platinum, palladium, and iridium, were prepared containing 1 at.% PM substituting for titanium, with the aluminium content remaining at 47.5 at.%. Hereafter, precious metal(s) are referred to as PM(s). A plain γ-TiAl alloy made with 52.5 at.% Al was included in the study for comparison.
Electrochemical tests
Potentiodynamic scans were conducted with an ACM AutoTafel potentiostat/galvanostat system using a conventional three-electrode system with a saturated calomel electrode (SCE). For each alloy system, tests were done in 5, 15, and 25 wt% HCl at room temperature to assess the effect of solution concentration on the corrosion rate. Scans were conducted with Grade 2 titanium, CP aluminium, plain aluminides (α2 + γ-TiAl), and aluminides with addition of precious metals.
The surfaces of the samples were ground to a 1200 grit SiC paper finish, degreased with methanol, rinsed with distilled water, and dried. The surface was prepared just prior to starting the polarization experiment to limit long-term oxidation. Before conducting the scan, each sample was immersed in the solution for approximately 60 minutes to obtain a stable corrosion potential. Potentiodynamic scans were done by scanning from -350 mV to +1000 mV versus the corrosion potential at a scanning rate of 10 mV.min-1.
Results and discussion
Electrochemical behaviour
The results of the different tests are shown in Table I, and the scans at different solution concentrations are shown in Figures 1 to 10. Table II shows the potential increase jump observed with increasing solution concentration (5, 15, 25 wt% HCl) after 60 minutes' exposure with reference to plain TiAl, and was established with the following calculations:

where:
ΔE = Potential increase difference
E5 = Corrosion potential in 5 wt% HCl solution
E15 = Corrosion potential in 15 wt% HCl solution
E25 = Corrosion potential in 25 wt% HCl solution
ETiAl = Corrosion potential of plain TiAl alloy
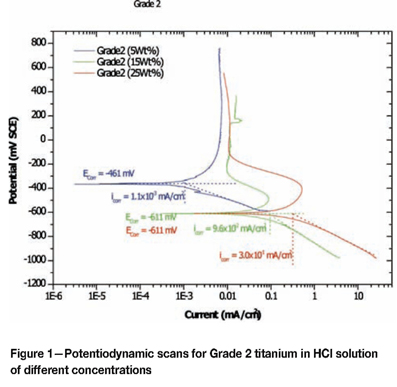
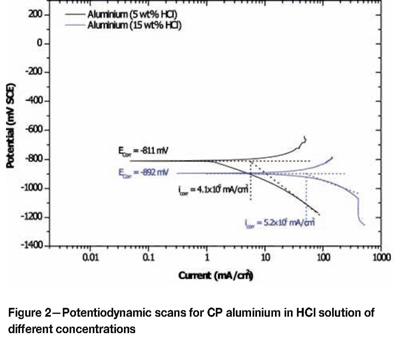
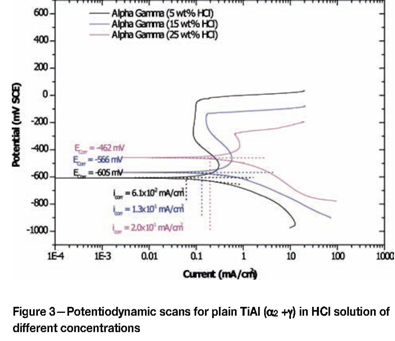
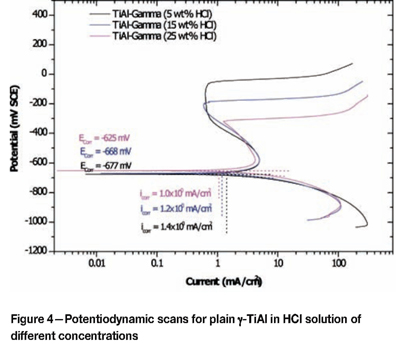
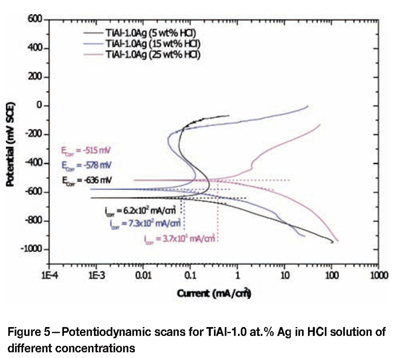
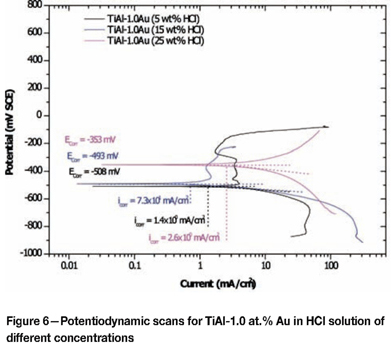
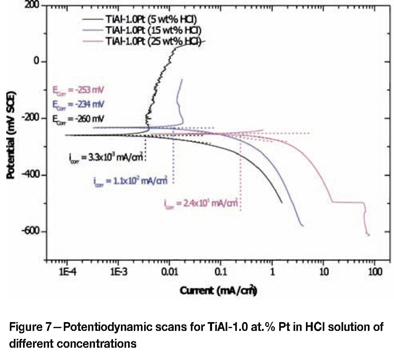
Effect of alloying addition on corrosion potential
Corrosion of Grade 2 titanium and CP aluminium
Results for Grade 2 Titanium are shown in Table II and Figures 1. Aluminium (Figure 2) was less noble than titanium, suggesting that aluminium was more susceptible to corrosion than titanium. In 25 wt % HCl solution, the sample was completely destroyed. Such a response was expected, because aluminium and its alloys are known to be susceptible to higher corrosion rates than steel and titanium in chloride solutions. Aluminium shows a pitting potential lower than the H+/H2 equilibrium in neutral electrolytes at 1M concentration34.
Corrosion of plain TiAl
TiAl with α2 + yphases in the microstructure
In the 5 wt% HCl solution, duplex titanium aluminide (Table I and Figure 3) had a lower corrosion potential than Grade 2 titanium and higher than CP aluminium, indicating that titanium aluminide was nobler than aluminium and less noble than Grade 2 titanium. Titanium aluminide gave the most positive Ecorr values in 15 wt% and 25 wt% HCl solutions. It appeared that in high-concentration solutions, duplex TiAl had a better resistance to corrosion. The high corrosion potential observed with titanium aluminide in the 25 wt% HCl solution was due to the combined contribution of titanium and aluminium, a result of passivation of the alloy surface, as was demonstrated in previous work on aluminides35. There is no data available for aluminium in 25 wt% HCl solution, because it could not stand the aggressiveness of the solution and was completely destroyed. The primary passivation zone range decreased with increasing concentration of HCl in solution. At all solution concentrations, the potentiodynamic scans indicated that there was pitting at potentials that decreased with increasing acid concentration. Alloy pitting was followed by a transpassive region that ended with a secondary passivation at current densities that were very close, irrespective of solution concentrations.
The increase of corrosion potential with increasing solution concentrations is believed to be the result of a strong and fast passivation reaction of the TiAl alloy in a more and more aggressive solution.
Plain TiAl with only γ phase in the microstructure
The potentiodynamic scan of single-phase titanium aluminide is shown in Figure 4. There was a high variation of corrosion potential as the solution concentration increased from 5 wt% HCl to 15 wt% HCl, and a small potential variation between the 15 wt% and 25 wt% HCl solutions, indicating that at higher solution concentrations, the corrosion potential was not influenced by the change in concentration. Overall, the scans gave corrosion potentials that were less noble than the corresponding potentials observed with duplex TiAl in solutions of the same concentration, suggesting that α2+γ-TiAl was more corrosion resistant than γ-TiAl. The low potentials observed with the γ-TiAl seemed to be the contribution of aluminium content, which was higher than in the duplex TiAl. Alloying of titanium with aluminium in a given solution was expected to result in a corrosion potential lower than the titanium corrosion potential and higher than the aluminium corrosion potential in the same solution. Lowering of corrosion potential with alloying is well documented for aluminium when it is alloyed with less noble elements, such as magnesium and zinc36.
The passivation region for γ-TiAl decreased with increasing solution concentration. The pitting potential also decreased with increasing solution concentration, and transpassivity occurred at potentials that decreased with increasing solution concentrations. Secondary passivation occurred at a current density that was almost the same, irrespective of the solution concentration.
Corrosion of duplex TiAl alloyed with precious metals
Overall, the addition of 1 at.% precious metals to duplex TiAl resulted in an increase of corrosion potentials, compared to plain duplex TiAl in solutions of same concentration. The results are discussed further.
TiAl alloyed with silver and gold
The potentiodynamic scans for TiAl-1.0 at.% Ag and TiAl-1.0 at.% Au at different solution concentrations are given in Figures 5 and 6. Overall, there was no improvement of the corrosion resistance by addition of silver compared to the plain TiAl alloy. Instead, the addition of silver shifted the corrosion potential to less noble values (Table II), indicating that modification of the cathodic process could not be achieved with silver addition to TiAl alloy. There was an increase in the corrosion potentials for TiAl alloyed with gold (Table II). As the solution concentration increased, the corrosion potential increased as well. The corrosion potential increase with increasing solution concentration occurred at higher values than for the plain TiAl in solutions of the same concentration. The increase of corrosion potential with increasing solution concentrations is believed to be the result of a combined action of a strong and faster passivation reaction of the TiAl alloy in an increasingly aggressive solution, and an increase in gold content on the surface. The values of Ecorr in the TiAl with gold suggested that the change in concentration from 5 wt% HCl to 15 wt% HCl impacted little on the corrosion potential (Table II).
TiAl alloyed with PGMs
The addition of PGMs to duplex TiAl resulted in an increase of the corrosion potential (Table I). Typical potentiodynamic scans are shown in Figure 7 for duplex TiAl alloyed with 1.0 at.% Pt. The addition of platinum shifted the corrosion potential to nobler values. These results showed that TiAl-1 at.% Pt will passivate spontaneously in 5, 15, and 25 wt % HCl solutions.
The corrosion potential in 25 wt% HCl was lower than in 15 wt% HCl owing to platinum being removed from the exposed surface of the TiAl samples and the relative amount of the two phases α and y changing, resulting in a decrease of the corrosion potentials. There was more γ-TiAl phase than α2 (Ti3Al), resulting in a higher aluminium content, although this explanation needs to be verified by conducting EDX and XPS on the surface of the samples and potentiodynamic scans on the α2-phase samples. From the data available, it is speculated that the corrosion potential of α2 (Ti3Al) was higher than that of y (TiAl), because of the higher titanium content.
However, a very large increase in corrosion potential (Table III) could result in the cathodic process falling in the transpassive region of the plain TiAl, and an increase in current density, corresponding to alloy dissolution. Whether the cathodic process of the TiAl alloyed with PGMs fell in the active, passive, or transpassive region of the plain TiAl was verified by comparing the corresponding addition scans together with the plain TiAl scans.
Cathodic process modification
The analysis of the results has shown that aluminium, with a very negative dissolution potential, was more susceptible to pitting corrosion than Grade 2 titanium. For other alloy systems36, alloying titanium with aluminium was expected to lead to a corrosion potential between the corrosion potentials of titanium and aluminium. The value of that corrosion potential will depend on the aluminium content in the TiAl alloy. From the data gathered with duplex TiAl (α2 + γ) and single-phase γ-TiAl, it appeared that the corrosion potentials of the duplex α2 + γ-TiAl were higher than those for single-phase γ-TiAl at all solution concentrations, indicating that γ-TiAl, with high aluminium content, would give lower corrosion potentials. It was also observed that the passivation region of plain duplex TiAl decreased with increasing solution concentration.
Thus, the introduction of precious metals in TiAl alloy resulted in a general improvement of corrosion resistance by shifting the corrosion potentials to nobler values. However, the cathodic modification will be achieved only:
- When the plain duplex TiAl has a small critical current density (icr) that will be easily exceeded by the current of the hydrogen cathodic reaction on TiAl with precious metal addition at the given passivation potential (Ep)
- If the passivation potential (Ep) of plain duplex TiAl is sufficiently negative to allow the cathodic component that has been introduced to change the corrosion potential (Ecorr) of the TiAl with precious metal to a value in the passive range that is more positive than Ep but less positive than the potential associated with the onset of transpassive processes (Efr)
- When the transpassive potential (Efr) of TiAl is sufficiently electropositive to allow a wide passive range.
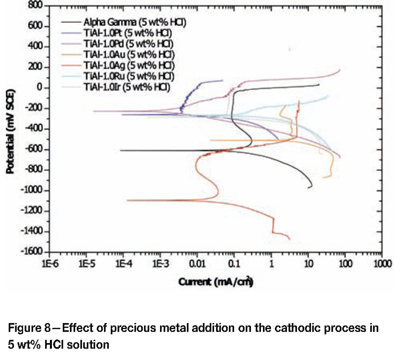
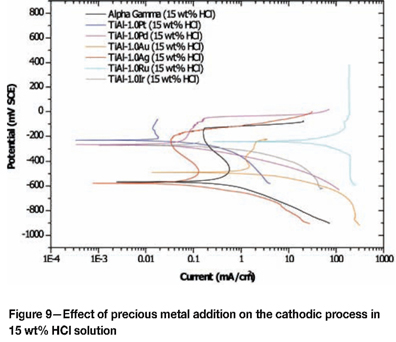
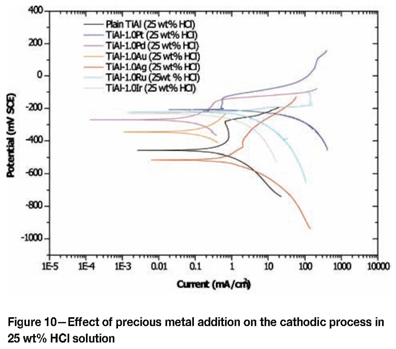
Figures 8 to 10 are the potentiodynamic scans of TiAl alloyed with precious metals superimposed on the potentio-dynamic scans of plain duplex TiAl. Table III shows the extension of the passive region in the 5 wt% HCl, indicating that the corrosion potentials of TiAl alloyed with PGMs fell in that range, and none of the scans intersected the scan of plain TiAl in the transpassive region (Figure 8), suggesting that TiAl alloyed with PGMs would spontaneously passivate in the 5 wt% HCl solution by modification of the cathodic process, except for Ru. A similar trend was observed in the 15 wt% HCl solution, where all the cathodic processes of TiAl alloyed with PGMs fell within the passivation region, without intersecting the scan of the plain TiAl scan in the active or transpassive regions (Figure 9). However, in the 25 wt% HCl solution, corrosion potentials of TiAl alloyed with precious metals all fell outside the passivation region (Figure 10). The cathodic scan of TiAl alloyed with silver, gold, and palladium intersected the plain TiAl scan in the active region, indicating that alloy dissolution would occur. The cathodic process of TiAl alloyed with platinum, and iridium intersected the plain TiAl scan in the transpassive region, indicating that alloy dissolution would occur. This behaviour of alloys in the 25 wt% HCl solution suggested that it would be very difficult to improve corrosion resistance of TiAl alloys by cathodic modification with some precious metals.



Conclusion
Titanium aluminide of composition Ti-47.5 at.% Al was prepared by melting Grade 2 titanium and CP aluminium with 1 at.% additions of gold, silver, platinum, palladium, ruthenium, and iridium. The corrosion behaviour of the alloyed titanium aluminide was investigated by conducting potentiodynamic scans in 5, 15, and 25 wt% HCl aqueous solution at room temperature and compared to CP aluminium, Grade 2 titanium, and plain titanium aluminide of duplex microstructure (α2 + γ-TiAl).
CP aluminium was very susceptible to corrosion and Grade 2 titanium did not exhibit any significant susceptibility to pitting. Plain titanium aluminide (α2 + γ) - TiAl was nobler than CP aluminium and less noble than titanium Grade 2 in all solution concentration.
Silver addition to titanium aluminide did not bring the corrosion potential Ecorr above that of plain titanium aluminide for all solution concentrations, indicating that silver addition could not improve the corrosion resistance. Gold addition to TiAl alloy brought a slight improvement, resulting in the active-passive state.
Overall, addition of platinum group metals improved corrosion resistance by increasing the open circuit potential to nobler values. Platinum, palladium, and iridium additions shifted the corrosion potential to the passive region of plain TiAl in 5 wt% and 15 wt% HCl solutions, suggesting that TiAl alloyed with these platinum group metals will passivate spontaneously by modification of the cathodic process. In 25 wt% HCl solution, the new corrosion potentials fell in the transpassive region as a result of the shift of the TiAl passivation region to lower potentials, leading to alloy dissolution by some of the platinum group metal alloys.
References
1. Lipsitt, H.A. Titanium aluminides - An overview. Materials Research Society Proceedings, vol. 39, 1984. pp. 351-365. [ Links ]
2. Banerjee, D. Ti3Al and its Alloys. Intermetallic Compounds, Principles and Practice, vol. 2. Westbrook, J.H and Fleischer, R.L. John Wiley, Chichester, England, 1995. pp. 91-131. [ Links ]
3. Huang, S. and Chesnutt, J.C. Gamma TiAl and its Alloys. Intermetallic Compounds, Principles and Practice, vol. 2. Westbrook., J.H and Fleischer, R.L.. John Wiley, Chichester, England, 1995. pp. 73-90. [ Links ]
4. Yamaguchi, M. and Inui, H. Al3Ti and its L12 variation. Intermetallic compounds, Principles and Practice, vol. 2. Westbrook., J.H and Fleischer, R.L. John Wiley & Sons Ltd, Chichester, England, 1995. pp. 73-90. [ Links ]
5. Djanarthany, S., Viala, J-C., and Bouix, J. An overview of monolithic titanium aluminides based on Ti3Al and TiAl. Materials Chemistry and Physics, vol. 72, no. 3, 2001. pp. 301-319. [ Links ]
6. Rivera-Denizard, O., Diffoot-Carlo, N., Navas, V., and Sundaram, P.A. Biocompatibility studies of human foetal osteoblast cells cultured on gamma titanium aluminide. Journal of Materials Science: Materials in Medicine, vol. 19, no. 1, 2008. pp. 153-158. [ Links ]
7. Zhang, B.B., Zheng, Y.F., and Liu, Y. Effect of Ag on the corrosion behaviour of Ti-Ag alloys in artificial saliva solutions. Dental Materials, vol. 25, 2009. pp. 672-677. [ Links ]
8. Bello, S.A., Maldonado, I., Rosim-Fachini, E., Sundaram, P.A., and Diffoot-Carlo, N. In vitro evaluation of human osteoblast adhesion to a thermally oxidized γ-TiAl intermetallic alloy of composition Ti-48Al-2Cr-2Nb (at.%). Journal of Materials Science: Materials in Medicine, vol. 21, 2010. pp. 1739-1750.
9. Verdonik, D.P., Darwin, R.L., and Williams, F.W. Assessment of aircraft collateral damage from dry chemical fire extinguishing agents. uS Navy Halon 1211 Replacement Program, Naval Research Laboratory, Washington DC, 1999. pp. 24-45. [ Links ]
10. Mackenzie, S. Overview of the Mechanisms of Failure in Heat Treated Steel Components, Failure Analysis of Heat Treated Steel Component. ASM International, Novelty, ohio, 2008, pp. 43-86. [ Links ]
11. Brossia, C.S. and Cragnolino, G.A. Effects of environmental and metallurgical conditions on the passive and localised dissolution of Ti-0.15%Pd. Corrosion, vol. 9, 2001. pp. 768-779. [ Links ]
12. Trufanov, A.A., Katsov, K.B., and Chervonyi, M.V. Effect of Ni and Mo additions on the corrosion-mechanical properties of Ti-Al alloy, Khlorvinil Production Organisation, Kaluga. Translatedfrom Fiziko-khimicheskaya Mekhanika Materialov, vol. 25, no. 3, 1989. pp. 28-32. [ Links ]
13. Schutz, R.W. Ruthenium enhanced titanium alloys. Platinum Metals Review, vol. 40, no. 2, 1996. pp. 54-61. [ Links ]
14. Hoar, T.P. Increasing the resistance of titanium to non-oxidising acids, influence of addition of platinum metals. Platinum Metals Review, vol. 4, no. 2, 1960. pp. 59-64. [ Links ]
15. Potgieter, J.H. Corrosion of passive alloys: Effect of noble metal additions. Shreir's Corrosion, vol. 4, no. 3, 2010. Elsevier, Amsterdam. pp. 2224-2249. [ Links ]
16. Potgieter, J.H. and Brookes, H.C. Corrosion behaviour of a high-chromium duplex stainless steel with minor additions of ruthenium in sulfuric acid. Corrosion, vol. 51, no. 4, 1995. pp. 312-320. [ Links ]
17. Olubambi, P.A., Potgieter, J.H., and Cornish, L.A. Corrosion behaviour of superferritic stainless steels cathodically modified with minor additions of Ru in sulphuric and hydrochlorioc acid solutions. Materials and Design, vol. 30, no. 5, 2009. pp. 1451-1457. [ Links ]
18. Monnartz, P. The study of the Fe-Cr alloys with particular regard to their solubility toward acids, Metallurgie, vol. 8, 1911. pp. 161-176. [ Links ]
19. Streicher, M.A. Alloying stainless steel with the platinum metals increased resistance to corrosion in acids. Platinum Metals Review, vol. 21, no. 2, 1977. pp. 51-55. [ Links ]
20. McGill, I.R. Platinum metals in stainless steels. Platinum Metals Review, vol. 34, no. 3, 1990. pp. 144-154. [ Links ]
21. Kim, Y.H. and Frankel, G.S. Effect of noble metal alloying on passivity and passivity breakdown of nickel. Journal of the Electrochemical Society, vol. 154, 2007. pp. 36-42. [ Links ]
22. Korb, L.J. and Olson, D.L. Corrosion. ASM Handbook, vol. 13, no. 9, 1987. pp. 375-498. [ Links ]
23. Potgieter, J.H., Heyns, A.M., and Skinner, W. Cathodic modification as a mean of improving the corrosion resistance of alloys. Journal of Applied Electrochemistry, vol. 20, 1990. pp. 711-715. [ Links ]
24. Van der Lingen, E. and Sandenbergh, R.F. The cathodic modification behaviour of ruthenium additions to titanium in hydrochloric acid. Corrosion Science, vol. 43, 2001. pp. 577-590. [ Links ]
25. Krasicka-Cydzik, E., Kierzkowska, A., and Glazowska, I. Behaviour of anodic layer in Ringer's solution on Ti-6Al-4V ELI alloy after bending. Archives of Materials Science and Engineering, vol. 28, no. 4, 2007. pp. 231-237. [ Links ]
26. Lee, C.P., Chen, Y.Y., Hsu, C.Y., Yeh, J.W., and Shih, H.C. Enhancing pitting corrosion resistance of AlxCrFe1.5MnNi0.5 high-entropy alloys by anodic treatment in sulphuric acid. Thin Solid Films, vol. 517, 2008. pp.1301-1305. [ Links ]
27. Cerny, M. and Filipek, J. Anodic-modified anticorrosive coatings. Acta Universitatis Agriculturae Mendelianae Brunensis, vol. 59, no. 5, 2011. pp. 23-30. [ Links ]
28. Satoh, H., Shimagori, K., and Kamikubo, F. The crevice corrosion resistance of some titanium materials: A review of the beneficial effects of palladium. Platinum Metals Review, vol. 31, no. 3, 1987. pp. 115-121. [ Links ]
29. Potgieter, J.H Alloys cathodically modified with noble metals. Journal of Applied Electrochemistry, vol. 241, 1991. pp. 471-482. [ Links ]
30. Diesel Marine International. Repair of T/C casings. Technical Services Bulletin, http://www.dmiglobal.com/downloads/Turbochargers.pdf [Accessed 7 March 2012] [ Links ].
31. Nordtest. http://nordtest.info/index.php/methods/item/combustion-by-products-corrosion-corrosivity-of-combustion-by-products-used-as-fill-around-carbon-steel-structures-nt-mat-001.html. [ Links ]
32. Lozitskii, L.P., Karpov, E.N., Kudryashov, B.Y., and Motrii, N.N. Corrosion resistance of gas turbine tested in a high-temperature gas flow. Kiev institute of Civil Aviation, 1974. Translated from Problemy Prochnosti, vol. 9, pp. 87-90. [ Links ]
33. Eliaz, N., Shemesh, G., and Latanasion, R.M. Hot corrosion in gas turbine components. Engineering Failure Analysis, vol. 9. 2002. pp. 31-43. [ Links ]
34. Szklarska-Smialowska, Z. Pitting corrosion of metals. NACE International, Houston, Texas, 1986. Ricker, R.E., Hall, D.E., and Fink, J.L., The effects of aqueous environments on the fracture behaviour of ductile nickel aluminide. Scripta Metallurgica et Materialia, vol. 24. 1990 pp. 291-296. [ Links ]
35. Vargel, C., Jacques, M., and Schmidt, M.P. Corrosion of Aluminium. Elsevier, Amsterdam, 2004. pp.81-109. [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN2225-6253.
This paper was first presented at the, Ferrous and Base Metals Development Network Conference 2012, 15-17 October 2012, Mount Grace Country House and Spa, Magaliesburg, South Africa..














