Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 spe Johannesburg jul. 2012
JOURNAL PAPER
Kinetics and thermodynamic parameters for the manufacturing of anhydrous zirconium tetrafluoride with ammonium acid fluoride as fluorinating agent
C.J. PretoriusI; W. du PlessisI; J.T. NelI; P.L. CrouseII
ISouth African Nuclear Energy Corporation Ltd. (Necsa)
IIUniversity of Pretoria
SYNOPSIS
More than 30 percent of the global demand for zircon (ZrSiC>4) is supplied by South Africa. A significant amount of the zircon is exported, and beneficiated products are then imported for industrial applications locally. Beneficiating the zircon locally could have a positive impact on the local market, since zircon is only one of many such cases. Ammonium acid fluoride serves as an alternative anhydrous fluorinating agent for zircon in the synthesis of several metal fluoridesi,2. It provides an effective dry fluorinating method and is easier to handle than hydrogen fluoride or fluorine gas. Zircon exposed to extreme plasma temperatures dissociates and becomes more reactive. The reaction of the plasma-dissociated zircon (ZrO2SiO2) with the ammonium acid fluoride (NH4FxHF, where x= 1 to 5) forms two main intermediate compounds (NH4)3ZrF7(s) and (NH4)2SiF6(s), the latter decomposing to form volatile products at relatively low temperatures, providing easy separation of the silicon and zirconium compounds. The ammonium zirconate compound decomposes to form zirconium tetrafluoride (ZrF4), which can be further manufactured into zirconium metal, to name but one product. Data on the kinetics of the reaction of ammonium acid fluoride with zircon and plasma-dissociated zircon, combined with the thermo-dynamic parameters of the reaction, is essential for the development of an industrial process for the production of a precursor for the manufacturing of zirconium metal, namely anhydrous ZrF4. Both the reaction kinetics and reaction parameters will be included in this study, as well as some proof that the reaction proceeds to ZrF4 on a small batch scale. If the exact reaction parameters can be pinned down, a wide spectrum of anhydrous metal fluorides can be synthesized through this fluorination route.
Keywords: Ammonium acid fluoride, zircon, plasma-dissociated zircon, zirconium tetrafluoride.
Introduction and literature
South African zircon reserves
South Africa is currently the second largest supplier of the world's zircon (ZrSiO4) demand and, next to Australia, also has the largest zircon reserves base in the world (30%)1. Despite this, there is not a single zirconium metal manufacturer in South Africa. A large portion of the zircon, obtained as a by-product from other mining activities, is exported as the raw material. Globally, only 5 to 10 percent of all zircon is destined for metal manufacturing. Zirconium metal is primarily used in the production of metal alloys, e.g. Zircaloy, which contains mainly zirconium and minor alloying additives such as tin and niobium. Zircaloy is used in water-cooled nuclear reactors as cladding for fuel rods because of its low thermal neutron cross-section, its high-temperature strength, and corrosion resistance in water.
Zirconium and hafnium always occur together in nature in a ratio of about 50:1. These two elements have similar chemical and physical properties, but in terms of thermal neutron absorbance cross-section, they are opposites. For this reason hafnium, as a strong neutron absorber, also finds application in nuclear reactors as control rods for regulation of nuclear activity. It is therefore imperative that hafnium is present only as trace amounts in zirconium metal and vice versa.
Another specification for nuclear-grade zirconium metal is that the levels of oxygen and hydrogen and nitrogen are extremely low to prevent brittleness of the Zircaloy tubes. A product that conforms to this requirement can be more efficiently manufactured by implementation of an anhydrous processing route.
Conventional methods for processing of zircon
Zircon is notoriously difficult to process because it is chemically inert and insoluble in most conventional acids under normal conditions. Current processing involves digestion of the mineral in large quantities of sodium hydroxide at temperatures above 800°C over long periods of time, or by conversion to zirconium tetrachloride via a carbo-chlorination process followed by liquid-liquid extraction using methyl isobutyl ketone. Both of these methods require vast amounts of energy and/or generate large liquid waste streams. A more cost-effective and environmentally-friendly process would therefore be a huge advantage.
Plasma-dissociated zircon (PDZ)
A plasma process was developed2 whereby zircon can undergo phase dissociation at temperatures above 1 700°C. Conversion of 95 percent is achieved within 15 ms contact time. The product consist of particles with a 120 m outside diameter consisting of a conglomerate of zirconia (ZrO2) crystals (< 1 m) embedded in an amorphous silica (SiO2) matrix. This phase dissociation of zircon results in a product (known as plasma-dissociated zircon (ZrO2-SiO2)) that is chemically more reactive than zircon and can be processed more efficiently.
Fluorination of PDZ
In order to develop a more efficient, cost-effective, and environmentally-friendly process for the beneficiation of South Africa's zircon reserves, a dry fluorination route is under investigation. Various methods for the production of zirconium tetrafluoride are being studied, one of which is fluorination with ammonium acid fluoride. Ammonium acid fluoride has been identified as an alternative fluorinating agent3,4 because of its low vapour pressure at room temperature (making it safer to handle) and lower cost compared to that of fluorine and hydrogen fluoride. Both ammonium bifluoride (NH4F-HF) and ammonium acid fluoride (NH4FxHF, x > 1) were used during the determination of the reaction kinetics of the fluorination of PDZ.
The reaction of ammonium bifluoride (and ammonium acid fluorides) with metal oxides yields intermediates known as ammonium fluorometallatess-?. In the case of PDZ, the intermediates (NH4)3ZrF7 and (NH4)2SiF6 are formed. At temperatures higher than the initial temperature at which the fluorination takes place, these intermediate compounds decompose to the corresponding metal fluoride and gaseous HF and NH38. The ammonium fluorosilicate compound is vapourized at a lower temperature^ than the zirconate and desilication is achieved in this wayio.
The stoichiometric reaction of PDZ with ammonium acid fluoride to produce ZrF4 can be described by the chemical equations below. The assumption is made that only the bound HF is involved in the dissolution of the oxides, i.e. two HF fluoride anions substitute for each O2-.
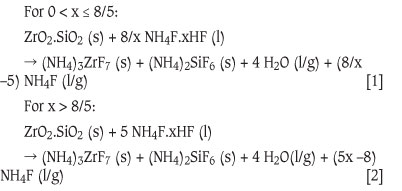
The phases, shown in parentheses, are dependent on the temperature regime one is working in. In general it is preferred to have the ammonium acid fluoride as a liquid to prevent mass transfer problems. The upper and lower temperature bounds are thus the melting and boiling points of the acid fluoride, which is determined by the value of x. The product NH4F is indicated in Equations [1] and [2] as liquid or gas. It should be noted that it decomposes thermally to form gaseous ammonia and hydrogen fluoride.
All of the products can be volatilized at temperatures below 400°C, leaving behind only ZrF4, the product obtained through decomposition of the ammonium heptafluorozirconate.
![]()
Silicon tetrafluoride, on the other hand, is a gas, thus the ammonium hexafluorosilicate decomposes to yield only gaseous products at a temperature of about 300°C, according to:
![]()
Experimental
PTFE containers each containing about 10 g of NH4F-HF or NH4F4.5HF were heated to the desired temperature for roughly one hour. Samples of approximately 1 g of ~94 percent PDZ were weighed and the exact mass was recorded. PDZ was added to the heated ammonium bifluoride or ammonium acid fluoride and lightly shaken/stirred for between 5 seconds and 60 minutes. To quench the reaction, a boric acid solution was added at time t and the mixture was rinsed with water to remove water-soluble reaction products. Boric acid reacts with the excess HF to form HBF4 which is also water-soluble. The remainder of the reaction mixture was dried, after which it was weighed and the exact mass of unreacted feedstock material was recorded. The experiment was repeated at different reaction temperatures (T > 125°C for NH4F-HF and T > 55°C for NH4F4.5HF). Curves of the conversion (α) vs time were plotted for every temperature. The data was fitted to a kinetic model and kinetic parameters were determined.
Results and discussion
Comparison of reactivity of NH4F^HF and NH4F^1.5HF
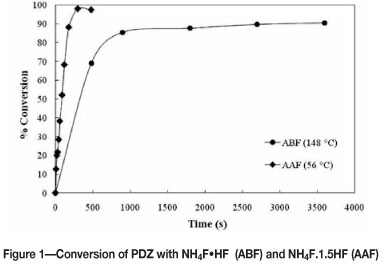
From Figure 1 it is evident that ammonium acid fluoride with higher HF content is more reactive and better conversion of PDZ is achieved within shorter time periods. Since NH4F.xHF with x > 1 is a liquid at room temperature, contact between the fluorinating agent and the feedstock material can be established at significantly lower temperatures than with ammonium bifluoride (i.e. for x = 1), which has a melting point of 125°C.
Kinetics of the reaction of PDZ with NH4F.1.5HF
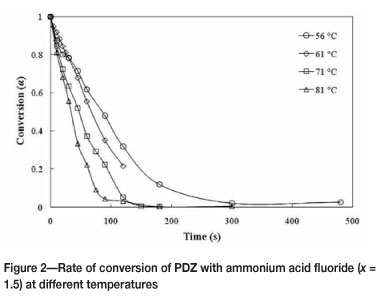
Near complete conversion of plasma-dissociated zircon was achieved within 5 minutes when NH4F.1.5HF was used as fluorinating agent. Furthermore, this was effected at temperatures below 100°C (Figure 2).
Model for determining kinetic parameters of fluori-nation reaction
The progressive conversion model is proposed for the reaction by which PDZ is converted to water-soluble reaction intermediates by means of ammonium acid fluoride. According to this model the reaction rate is controlled by the chemical reaction on the surface of the particle and the role of diffusion is negligible. It is also assumed that all PDZ particles are spherical and of the same size. The rate of the fluorination reaction is therefore directly proportional to the surface area of the particle, and thus

where
![]()
pB is the density of PDZ, R is the initial radius of one PDZ particle, α is the conversion of the PDZ, and k is the reaction constant in kg.s-1.m-2. Integration of this model gives
![]()
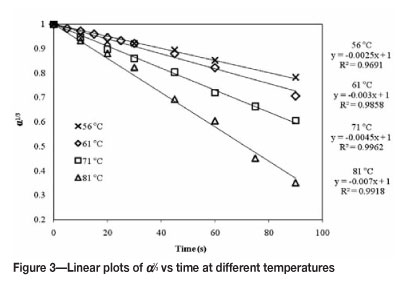
A plot of α against t resulted in a straight line with intercept through the t-axis at t = τ (Figure 3).
Values for the reaction constants at each temperature were determined and the Arrhenius parameters calculated for the reaction. The Arrhenius plot is shown in Figure 4.
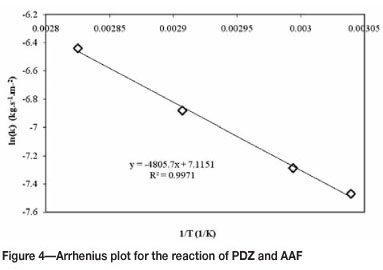
The activation energy (Ea) calculated for this reaction is 40 kJ.mol-i. This relatively low value for activation energy indicates that the reaction progresses easily and the requirement for input energy is low.
Decomposition of reaction intermediates
A horizontal tube furnace fitted with a vacuum pump in the off-gas line was used to manufacture gram-scale amounts of ZrF4 by reaction of PDZ with ammonium bifluoride. The temperature of the tube furnace was programmed to heat the reaction mixture stepwise. This was to ensure maximum conversion of PDZ in the fluorination step and complete decomposition of the subsequently formed reaction intermediates. A constant flow of nitrogen was introduced in the tube and the system was kept at a pressure below atmospheric to afford anhydrous conditions. In doing so, the water formed during the fluorination reaction can be removed. This is imperative for the production of 'dry' and essentially oxygen-free ZrF4 as precursor for the plasma reduction process.
A calculation was made in order to determine the amount of product that should form. The calculation (Table I) was based on the assumptions that the purity of the PDZ is 94% (the remainder being zircon), only 5% of the zircon reacts to form ZrF4, and the reaction of PDZ to form ZrF4 is assumed to be complete.
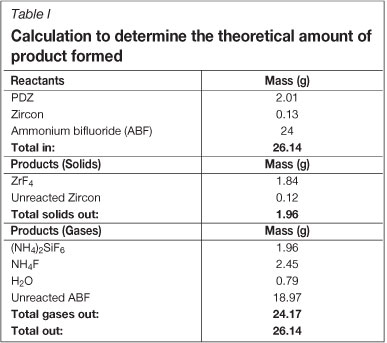
The product mass collected from the reaction vessel was approximately 1.8 g, which is more than 90 percent recovery, according to the theoretically calculated amount.
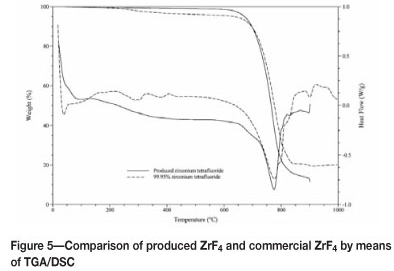
The product was analysed by means of scanning electron Microscopy with energy dispersive X-ray spectroscopy (SEM-EDAX), thermogravimetric differential scanning calorimetry (TG/DSC), and X-ray diffraction. SEM-EDAX analysis confirmed, on an elemental scale, that the major elements present in the product are Zr and F. TG/DSC confirmed that ZrF4 was synthesised with less than 15% impurities, i.e. the residue remaining after sublimation of ZrF4. Another method of identification through TG/DSC was to compare a known sample of ZrF4 (>99% purity) with the product. Through this comparison, it was shown the product is indeed ZrF4 due to the overlapping of the two TG/DSC curves. The TG/DSC curves are shown in Figure 5.
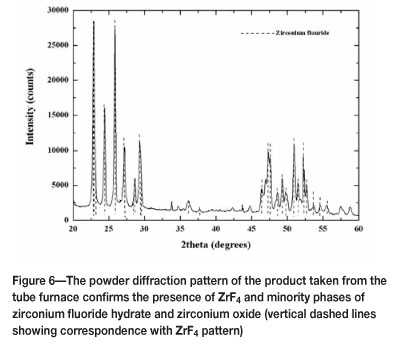
X-ray diffraction (Figure 6) also confirmed the presence of ZrF4 as the major peak. The impurities were identified, also by X-ray diffraction, as ZrO2 and a zirconium tetrafluoride hydrate, which may have formed as a result of water and/or oxygen present in the system.
Processing of zircon via the ammonium acid fluoride route
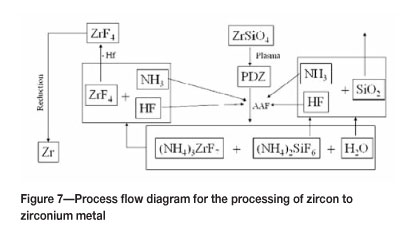
The envisaged process for the production of ZrF4, and eventually zirconium metal, is illustrated in Figure 7. Also note the potential for recycling of the reaction products in the form of NH3 and HF. These compounds form NH4F at lower temperatures. By adjustment of the HF concentration, ammonium acid fluorides can be re-introduced as the fluorinating agent.
Conclusions
The kinetics of the reaction of plasma-dissociated zircon with ammonium acid fluoride were successfully determined and the activation energy was calculated to be 40 kJ.mol-1. This value for activation energy is relatively low, which serves as an indication that the reaction progresses with ease and the requirement for energy input will therefore be low. The process was also successfully conducted on a scale of ~2 g ZrF4. When considering that energy consumption is a significant factor in validation of the techno-economical aspects of a process, this result may be seen as very positive for possible future commercialization.
Acknowledgements
Financial assistance from the Advanced Metals Initiative of the Department of Science and Technology through the New Metals Development Network, as well as assistance from Necsa colleagues, is gratefully acknowledged.
References
1. Omphemetse, M. An overview of South Africa's zircon industry and the role of BEE. Directorate: Mineral Economics, Department of Minerals and Energy, Pretoria, 2007. [ Links ]
2. Nel, J. Treatment of a Chemical, C.I.P. Atomic Energy Corporation of South Africa Limited, W09616903, South Africa, 1995. [ Links ]
3. Nel, J.T., Du Plessis, N., Le Roux, J.P., and Retief, W.L Treatment of zirconia-based material with ammonium bifluoride, South African Nuclear Energy Corporation Ltd., W02011013085. South Africa, 2011. [ Links ]
4.Nel, J.T., Du Plessis, N., Le Roux, J.P., and Retief, W.L. Treatment of Minerals, South African Nuclear Energy Corporation Ltd., W02011030301. South Africa, 2011. [ Links ]
5. Mel'nichenko, E.I., Krysenko, G.F., Epov, D.G., and Cvsyannikovex, A.A. Reaction of zirconium concentrate with ammonium hydrogen fluoride. Russian Journal of Applied Chemistry, vol. 67, no. 5, 1994. pp. 659-662. [ Links ]
6. Mikhailov, M.A., Epov, D.G. and Rakov, E.G. Thermodynamics of the dissociation of ammonium fluorozirconates and fluorohafnates. Russian Journal of Inorganic Chemistry, vol. 18, no. 1, 1973. pp. 56-58. [ Links ]
7. Onishi, M., Kohgo, T., Amemiya, K., Nakazato, K., Kanamoi, H., and Yokota, H. Thermal and mass analyses of fluorination process with ammonium bifluoride. Journal of Non-crystalline solids, vol. 161, 1993. pp. 10-13. [ Links ]
8. Kinsman, B.E. and Hanney, R. Preparation and purification of metal fluorides for crystals and glasses. Advanced Materialsfor Optics and Electronics, vol, 5. 1995.pp.109-115. [ Links ]
9. Mel'nichenko, E.I., Krysenka, G.F., Epov, D.G., and Marusora, E.Y. Thermal properties of (NH4)2SiF6. Russian Journal of Inorganic Chemistry, vol. 49, no. 12, 2004. pp. 1803-1806. [ Links ]
10. Mel'nichenko, E.I., Epov, D.G., Krysenka, G.F., Ovisyannikova, A.A., and Maslennikova, I.G. Desiliconization process in the processing and enrichment of mineral raw materials with ammonium hydrodifluoride. Russian Journal of Applied Chemistry, vol. 69, no. 8, 1996. pp. 1107-1110. [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2012. SA ISSN2225-6253. This paper was first presented at the ZrTa2011 New Metals Development Network Conference, 12-14 October 2011, Mount Grace Country House & Spa, Magaliesburg.














