Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 spe Johannesburg jul. 2012
JOURNAL PAPER
Manufacturing of anhydrous zirconium tetrafluoride in a batch reactor from plasma-dissociated zircon and ammonium bifluoride
M.M. Makhofane; J.L. Havenga; J.T. Nel; W. du Plessis; C.J. Pretorius
The South African Nuclear Energy Corporation Ltd. (Necsa)
SYNOPSIS
Anhydrous zirconium tetrafluoride can be used as a precursor for the manufacturing of nuclear-grade zirconium metal. This can be done by fluorinating plasma-dissociated zircon with ammonium bifluoride in a batch reactor. This paper describes the batch process. It was proved that anhydrous zirconium tetrafluoride can be manufactured by this route as confirmed by X-ray diffraction. The process shows potential for scaling up to the kilogram scale and possibly the tonnage scale.
Keywords: Zirconium tetrafluoride, zircon, plasma-dissociated zircon, PDZ.
Introduction
Anhydrous zirconium tetrafluoride can be used as a precursor for the production of nuclear-grade zirconium metal by reduction of the tetrafluoride with magnesium or calcium metal1,2. High-purity zirconium tetrafluoride is also one of the major constituents of the ZBLAN glass (ZrF4-BaF2-LaF3-AlF3-NaF) which is used in optical fibre applications3.
Various methods are used for the production of zirconium tetrafluoride, including halogen exchange, where zirconium tetrachloride is reacted with hydrogen fluoride gas at 300°C to produce zirconium tetrafluoride3-5. Large quantities of zirconium tetrafluoride can be produced by adding concentrated (40 per cent) hydrofluoric acid to a concentrated (65 per cent) nitric acid solution of zirconium to precipitate zirconium tetrafluoride monohydrate. The precipitated monohydrate is dried and treated with hydrogen fluoride gas at 450°C to form anhydrous zirconium tetrafluoride5. Zirconium tetrafluoride can also be produced by hydrofluorination of zirconium dioxide at 25°C followed by sublimation in hydrogen fluoride gas3 at 825°C. Zirconium dioxide or zirconium tetrachloride can also be reacted with ammonium bifluoride at 200°C to form ammonium heptafluorozirconate [(NH4)3ZrF7], which is thermally decomposed to form zirconium tetrafluoride and ammonium fluoride3,6,7 at approximately 450°C.
The prime objective of this work was to produce zirconium tetrafluoride on a kilogram scale that can be used for subsequent sublimation and plasma reduction to zirconium metal. Zirconium tetrafluoride was prepared in a batch reactor by reacting plasma-dissociated zircon (PDZ, ZrO2.SiO2) with ammonium bifluoride (NH4HF2) according to the method described by Nel et al8. It was assumed that zirconium tetrafluoride was produced according to Equations [1] to [4]. Fluorination of PDZ occurs at approximately 180°C (Equation [1]). This is followed by a stepwise thermal decomposition of the ammonium fluorozirconate, at 300°C (Equation [2]), 350°C (Equation [3]), and 400°C (Equation [4])6.
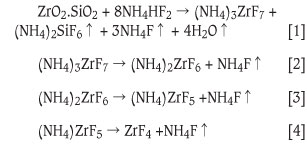
Experimental
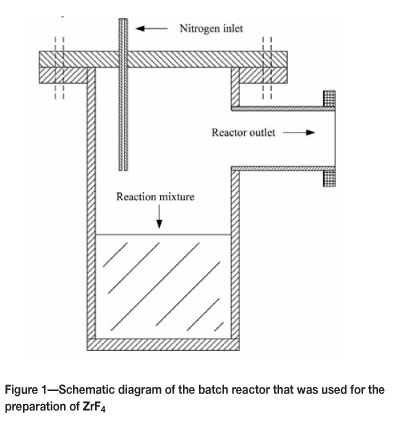
The batch reactor is schematically presented in Figure 1. This stainless steel reactor was 200 mm high and had a diameter of 100 mm. The reactor was externally heated by a 2 kW heating element. A thermocouple was used to monitor the internal temperature of the reactor, and the rate of heating and soaking time was electronically controlled. The reactor lid was fitted with a nitrogen inlet to purge the reactor and the downstream system with nitrogen, which would act as a carrier gas for the gaseous reaction products as well as to maintain an inert atmosphere. The nitrogen purge rate was approximately 0.9 kg/h.
The process flow diagram of the system is presented in Figure 2. The off-gases from the reactor were passed through a cold trap, where the gaseous reaction compounds solidified upon contact with the cold surfaces of the trap. The cold trap consisted of a water-cooled coil. The temperature of the water was at room temperature (approximately 18°C). Any fine particles that were carried further downstream, were removed by a cyclone. The off-gas was monitored in-line by Fourier Transform Infrared Spectrometry (FTIR). All the off-gases were further scrubbed by a HF scrubber and KOH scrubber before being released to the atmosphere.
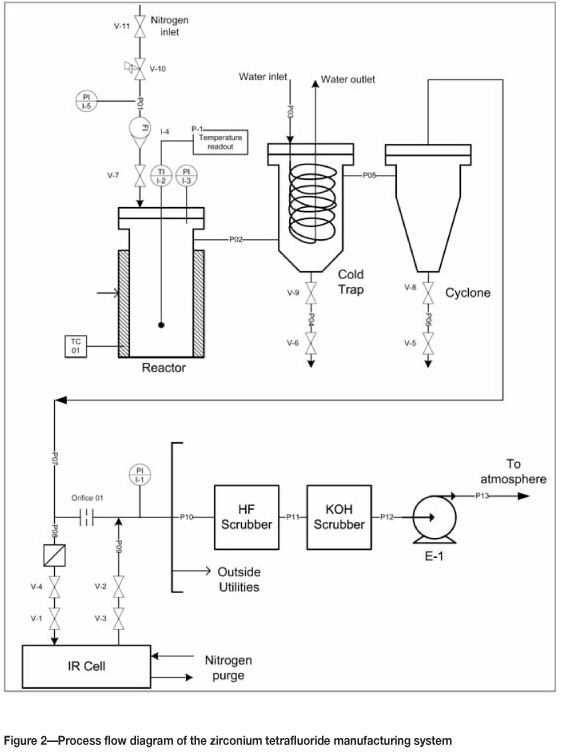
ZrF4 was prepared batchwise by loading a stoichiometric mixture of PDZ and ammonium bifluoride into the reactor. The PDZ was produced at Necsa and the ammonium bifluoride (purity >99 %) was obtained from Sigma Aldrich. The total mass of the mixture was about 400 g. The lid of the reactor was closed and the system was continuously purged with nitrogen for the full duration of the experiment. The reactor was heated to 150°C and kept at this temperature for 30 minutes. It was then heated to 280°C and kept there for 30 minutes. The temperature was then increased to 380°C and kept there for 30 minutes. These temperature steps correspond more or less to the temperatures of the reactions in Equations [1] - [4]. After the experiment, the reactor cooled to room temperature and was opened. The product was collected from the bottom of the reactor. The cold trap was also opened and a white fluffy type of product was collected from the cold finger and the cyclone. The product from the reactor and the cold trap were analysed by X-ray diffraction (XRD). No attempt was made to do an accurate mass balance or to analyse the product for impurities. This will be the purpose of further optimization experiments.
Results and discussions
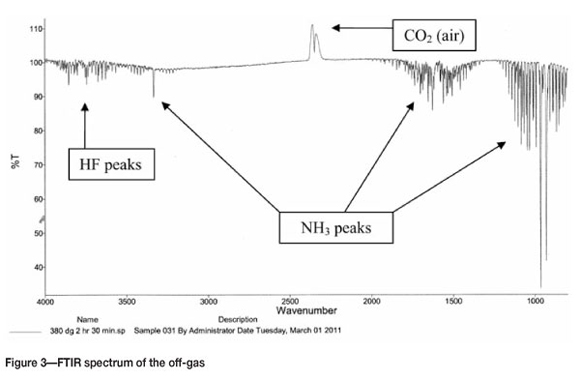
Qualitative analysis of the off-gas during the experiment by FTIR showed that hydrogen fluoride gas and ammonia were detected. A typical FTIR spectrum of the off-gas is presented in Figure 3. The spectrum clearly indicates the presence of hydrogen fluoride and ammonia, which are formed by the thermal decomposition of ammonium fluoride according to Equation [5]:
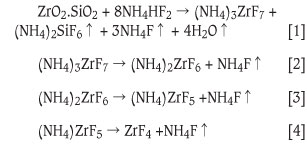
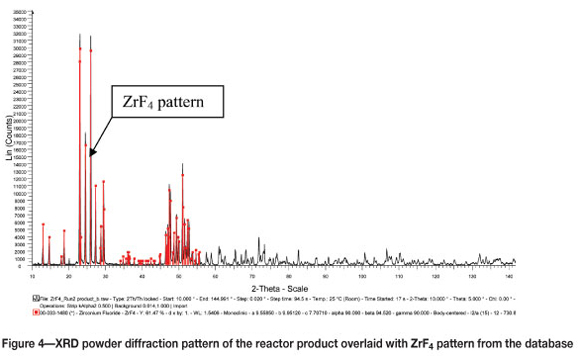
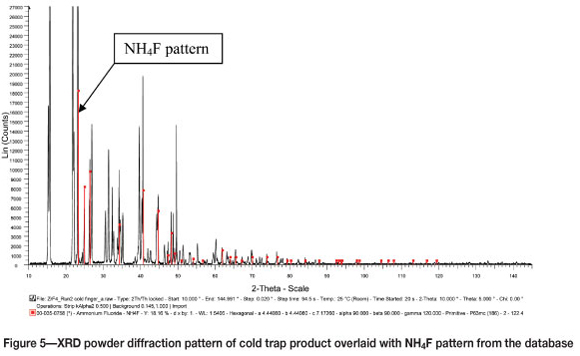
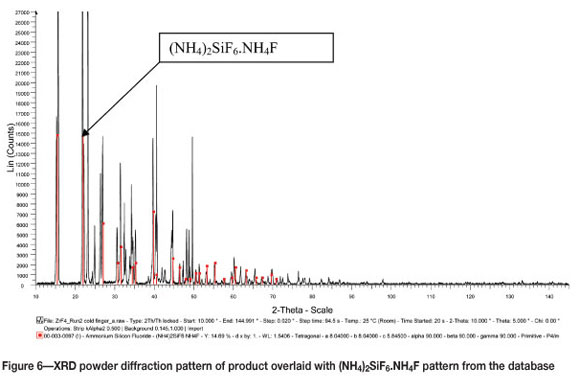
XRD analysis of the product that was collected at the bottom of the reactor showed that the reaction product was indeed anhydrous zirconium tetrafluoride, as expected (Figure 4). The white fluffy product collected from the cold finger was identified by XRD as a mixture of NH4F (Figure 5) and (NH4)2SiF6.NH4F (Figure 6).
Conclusion
Anhydrous zirconium tetrafluoride can be manufactured successfully in a batch reactor from a mixture of plasma-dissociated zircon and ammonium bifluoride. This was achieved on a scale of several hundred grams, and the process promises to be scalable to several kilograms or maybe even to tonnage scale. Several optimization experiments are planned for the future, including an accurate mass balance, the yield of the reaction, energy consumption, and purity of the reaction product.
Acknowledgements
The authors would like to thank the Advanced Metals Initiative (AMI) of the Department of Science and Technology (DST) for the funding of this project, the South African Nuclear Energy Corporation Ltd. (Necsa) for providing equipment, laboratory space, and performing the analysis, and the colleagues and co-workers that helped in making this experiment a success.
References
1. Wilhelm, H.A. and Walsh K.A. Preparation of zirconium tetrafluoride. US Patent 2635037. 14 April 1953. [ Links ]
2. Barnes, D.E. et al. Newnes' Concise Encyclopaedia of Nuclear Energy. George Newnes, London, 1962. p. 882. [ Links ]
3. Nielsen, R.H. et al. Ullmann's Encyclopedia of Industrial Chemistry, vol. 28. Zirconium and Zirconium compounds. Fifth Completely Revised Edition, New York. 1996. pp. 559-560. [ Links ]
4. Wilhelm, H.A. and Walsh, K.A. Preparation of zirconium tetrafluoride. US Patent 2602725. 08 July 1952. [ Links ]
5. Nielsen, R.H. et al. Kirk-Othmer Encyclopedia of Chemical Technology, vol. 26. Zirconium and Zirconium Compounds, 5th ed., New Jersey, 2007. p. 643. [ Links ]
6. MacFarlane, D.R., Mineely, P.J., and Newman, P.J. Synthesis of zirconium tetrafluoride using ammonium bifluoride melts, Journal of Non-Crystalline Solids, vol. 140, 1992. pp. 335-339. [ Links ]
7. Braglia, M. et al. Different fluorination processes with ammonium bifluoride and their effects on fluorozirconate glass. Materials Research Bulletin, vol. 24, 1989. pp. 661-669. [ Links ]
8. Nel, J.T. et al. Fluorinating zircon to zirconium tetrafluoride. RSA Patent ZA 2009/05297, 29 July 2010. [ Links ]
©The Southern African Institute of Mining and Metallurgy, 2012. SA ISSN2225-6253. This paper was first presented at the ZrTa2011 New Metals Development Network Conference, 12-14 October 2011, Mount Grace Country House & Spa, Magaliesburg.














