Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 spe Johannesburg Jul. 2012
TRANSACTION PAPER
Electrochemical studies of Fe-21Cr-1Ni duplex stainless steels with 0.15 wt% ruthenium at different temperatures
O.A. OlaseindeI, II, III; J.W. van der MerweI, II, III; L.A. CornishI, II, III; L.H. ChownI, II, III; P.A. OlubambiIII, IV
ISchool of Chemical & Metallurgical Engineering, University of the Witwatersrand
IIDST/NRF Centre of Excellencefor Strong Materials, University of the Witwatersrand
IIIAfrican Materials Science & Engineering Network
IVTshwane University of Technology, Pretoria, South Africa & Federal University of Technology, Nigeria
SYNOPSIS
The 2101 lean duplex stainless steel has wide potential application in storage, heat exchangers, and in the oil and gas industries. This work investigates the electrochemical behaviour of 2101 duplex stainless steel with an addition of 0.15 wt% ruthenium, using potentiodynamic techniques in 1M H2SO4 at 25°, 40°, 60°, and 80°C. The microstructures of samples were characterized using optical metallography and scanning electron microscopy.
The results showed that the ruthenium addition moved the corrosion potential of alloy 2101 to a more positive potential. All samples containing ruthenium displayed longer passive regions at 25°C and 80°C compared to those without ruthenium. Alloys without ruthenium had higher critical current density (?'crit) values when compared to the alloys with ruthenium. Ruthenium additions decreased the passive current densities and inhibited anodic dissolution. At room temperature the corrosion rate of alloys with ruthenium was lower than these without ruthenium.
Keywords: Duplex stainless steels, Fe-21Cr-1Ni, 2101LDX stainless steel, ruthenium addition, corrosion behaviour, passivation, microstructure.
Introduction
Duplex stainless steels, which are also referred to as ferritic-austenitic stainless steels, have the beneficial properties of both ferritic and austenitic steels. They contain high chromium contents and molybdenum. The high strength of the material enables the use of thinner gauges in a variety of applications such as tanks, pressure vessels, piping, and transportation, thus providing cost savings1. Lean duplex stainless steel 2101 has been used to build liquid storage tanks2.
Duplex stainless steels have previously been alloyed with platinum group metals (PGMs), such as palladium and ruthenium3-5. It has been reported that small ruthenium additions can significantly improve the corrosion resistance of the duplex stainless steels, displacing their open-circuit corrosion potential towards more positive values in HCl and H2SO4 solutions compared to austenitic or ferritic stainless steels6. The reduction in corrosion and critical current densities indicates that ruthenium inhibits the anodic dissolution of the cathodically modified alloys?. The following criteria must be met for successful cathodic alloying: the base alloy must have a small critical current density; the passivation potential of the alloy must be sufficiently negative to allow the cathodic component that has been introduced to change the corrosion potential; and the transpassive potential of the base alloy must be sufficiently electropositive to allow a wide passive range?. These conditions are satisfied in non-oxidizing acids by stainless steel, chromium-based alloys, and titanium based alloys, while the PGMs have the high exchange current density for hydrogen evolution8.
The addition of ruthenium to duplex stainless steels has been shown to have a beneficial effect on their corrosion resistances However, there has not been previous research work on the cathodic modification of lean 2101 duplex stainless steel. This work investigates the electrochemical behaviour of 2101 lean duplex stainless steel with 0.15 wt% ruthenium in aqueous sulphuric acid solutions at temperatures from 25-80°C.
Experimental procedure
The experimental 2101 lean duplex stainless steel (2101LDX) was obtained from the Stainless Steel Association of South Africa. The 2101 lean duplex stainless steel containing 0.15 wt% ruthenium (2101LDX40.15Ru) was produced using an arc melting furnace, then solution-annealed at 1 080°C for 90 minutes and water-quenched. The specimens for metallographic examination were cut using a StruersTM Secotom-10 cutting machine, wet ground on SiC papers, then polished using alumina suspensions of 3 and 1 pm. The duplex structure of the samples was revealed using 40 g NaOH in 100 ml of distilled water, according to ASTM Standard A923-06io. The microstructure was examined on a scanning electron microscope (SEM) at the University of Pretoria. EDS (energy-dispersive X-ray spectroscopy) analysis was done to determine the overall alloy and phase compositions, and averages of five different areas in each of the phases and of the overall sample composition were calculated.
Representative samples were cold-mounted in epoxy resin for the corrosion tests. The samples were ground on SiC papers, degreased with ethanol, and washed with distilled water. The size of the areas to be exposed to the corrosive solution were measured.
Solutions of 1M H2SO4 were prepared using analytical grade chemicals in distilled water. Electrochemical tests were performed using an AutolabTM Potentiostat (computer-controlled PGSTAT20) with the general-purpose electrochemical software (GPES) Version 4.9. Electrochemical studies were conducted using a conventional electrochemical cell, a graphite electrode, a silver/silver chloride reference electrode in saturated potassium chloride (KCl), and a working electrode.
The potentiodynamic polarization responses of the alloys were investigated in naturally aerated 1M H2SO4 solution at temperatures of 25, 40, 60, and 80°C. Polarization curves were measured at a scan rate of 2 mV.s-i starting from -500 mV to 1 500 mV with respect to open-circuit potential. The temperature was measured with a thermometer and maintained by immersing the cell in a water bath. All tests were carried out in triplicate, and good reproducibility was observed.
Results
The secondary electron image (SEI) obtained on the SEM and representative EDS analysis spectra of the phases of 2101 duplex stainless steel are presented in Figure 1. There were two distinct phases: austenite in a matrix of ferrite. The average EDS analyses for the phases are shown in Table I. Austenite had the highest content of nickel and manganese in both 2101LDX and 2101LDX+0.15Ru steels, whereas ferrite had the highest chromium and molybdenum content in 2101LDX. In 2101LDX+0.15Ru, the chromium was higher in ferrite and the molybdenum was the same in both ferrite and austenite. EDS could not be used to determine the ruthenium content because it was below 1 wt% Ru.
Figure 2 shows the SEM micrograph of 2101LDX+0.15Ru with representative EDS spectra of the phases. The austenite phase had the highest nickel content, and the ferrite phase had the highest chromium content.
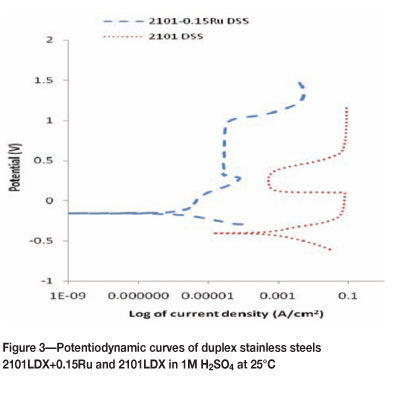
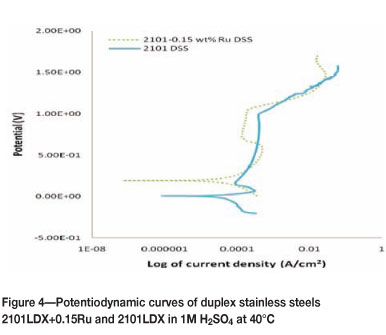
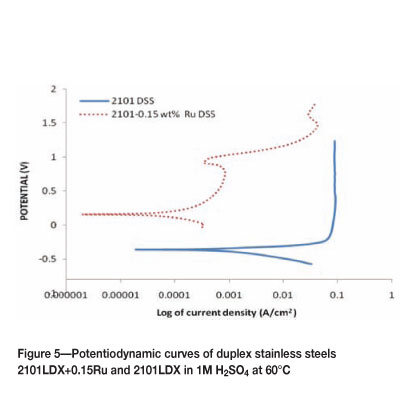
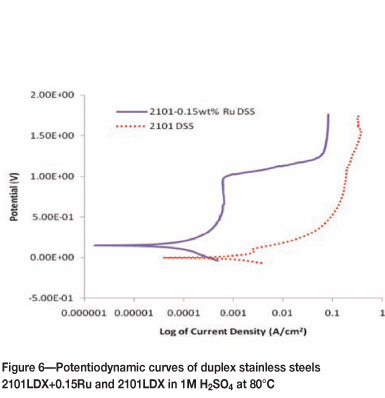
Figures 3 to 6 show the potentiodynamic polarization responses of 2101LDX and 2101LDX+0.15Ru in naturally aerated 1M H2SO4 solution at temperatures of 25, 40, 60, and 80°C respectively. The electrochemical data is summarized in Table II.
25°C
The addition of ruthenium to 2101 shifted the corrosion potential to a less negative value (Figure 3). The icrit value was greatly reduced in 2101LDX+0.15Ru. There was a longer passive region in 2101LDX+0.15Ru.
40°C
Figure 4 shows the effect of 1M sulphuric acid on 2101LDX and 2101LDX+0.15Ru at 40°C. Although the corrosion potential of 2101LDX+0.15Ru moved towards a more positive value, 2101 had a longer passive region than 2101LDX+0.15Ru, thus the corrosion rate of 2101 duplex stainless steel was lower.
60°C
The potentiodynamic polarization curve for 2101LDX and 2101LDX+0.15Ru in 1M sulphuric acid solution at 60°C is shown in Figure 5. The addition of ruthenium shifted the corrosion potential towards a more noble value and the critical current density was also reduced. There was good passivation of the 2101 duplex stainless steel.
80°C
Figure 6 shows the potentiodynamic results of 2101LDX and 2101LDX+0.15Ru in 1M H2SO4 at 80°C. The corrosion potential shifted towards a more positive value for samples with ruthenium than samples without ruthenium. For the two samples in the solution at 80°C, no active passivation was observed.
Discussion
The corrosion potential was lower for 2101LDX+0.15Ru in 1M sulphuric acid at 25°C than at other temperatures. The corrosion potentials for 2101LDX+0.15Ru at 40°C, 60°C, and 80°C were very similar. There was an increase in passive current density with temperature from 25°C to 60°C. The passive region became smaller with increase of temperature up to 60°C. No passivity was observed at 80°C.
The anodic behaviour of 2101LDX in 1 M sulphuric acid was very different at 25°C, 40°C, 60°C, and 80°C. At 60°C, 2101LDX had a lower passive current density than at other temperatures. There was active dissolution of 2101LDX sample at 25°C. No passivity was observed at 80°C.
According to the literatures, addition of ruthenium to stainless steel should move the corrosion potential of the steel towards a more noble potential. This was observed in the present investigation. The addition of ruthenium also lowered the critical current density.
Corrosion rate of the samples increased as the temperature increased from 25°C to 80°C for 2101LDX+0.15Ru. This could be due to the reduction in the passive region with temperature, and also a reduction in passive current density.
Ruthenium was observed to lower the corrosion rates of 2101 duplex stainless steel at room temperature. The samples with ruthenium showed a passive region at the lower temperatures of 25°C and 40°C. Ru and Ni act as blocking agents, which decrease the dissolution rates of Cr and Fe and therefore increase the probability of a stable passive layer forming?.
Conclusions
![]() The addition of ruthenium to 2101 duplex stainless steel shifted the corrosion potential to more noble values in the temperature range 20-80°C
The addition of ruthenium to 2101 duplex stainless steel shifted the corrosion potential to more noble values in the temperature range 20-80°C
![]() At lower testing temperatures, the addition of ruthenium lowered the critical current density
At lower testing temperatures, the addition of ruthenium lowered the critical current density
![]() Addition of ruthenium decreased the passive current densities of 2101 duplex stainless steel
Addition of ruthenium decreased the passive current densities of 2101 duplex stainless steel
![]() The passive current density increased with temperature in 2101LDX+0.15Ru samples
The passive current density increased with temperature in 2101LDX+0.15Ru samples
![]() There was a decrease in the passive region of 2101LDX+0.15Ru as the temperature increased.
There was a decrease in the passive region of 2101LDX+0.15Ru as the temperature increased.
References
1. International Molybdenum Association. Practical guidelines for the fabrication of duplex stainless steels. 2nd edn. www.imoa.info. 2009. [ Links ]
2. Outokumpu. www.outokumpu.com accessed May 2011. [ Links ]
3. Streicher, M.A. Alloying stainless steels with the platinum metals. Platinum Metals Review, vol. 21, 1977. pp. 51-54. [ Links ]
4. Potgieter, J.H. Alloys cathodically modified with noble metals. Journal of Applied Electrochemistry, vol. 21, 1991. pp. 471-482 [ Links ]
5. Potgieter, J.H. and Brookes, H.C. Duplex stainless steel with minor additions of ruthenium in sulfuric acid. Corrosion, vol. 51, 1995. pp. 312-320. [ Links ]
6. Potgieter, J.H. Effect of minor ruthenium additions on the corrosion behaviour of duplex stainless steels in sulfuric acid. Suid.-Afrikaanse, Tydskrif vir Natuurwentenstap en Tegnologie, vol. 46, 1993. pp. 3-4. [ Links ]
7. Myburg, G., Varga, K., Barnard, W.O., Baradlai, P., Tomcsany, I.L., Potgieter, J.H., Louw, C.W., and van Staden M.J. Surface composition of Ru containing duplex stainless steel after passivation in non-oxidizing media. Applied Surface Science, vol. 136, 1998. pp. 29-35. [ Links ]
8. Potgieter, J.H., Heynes, A.M., and Skinner, W. Cathodic modification as a means of improving the corrosion resistance of alloys. Journal of Applied Electrochemistry, vol. 20, 1990. pp. 711-715. [ Links ]
9. Potgieter J.H., Barnard W.O., Myburg, G., Varga, K., Baradlai, P., and Tomcsany, I.L. Corrosion behaviour of duplex stainless steels containing minor ruthenium additions in reducing acid media. Journal of Applied Electrochemistry, vol. 26, 1996. pp. 1103-1110. [ Links ]
10. ASTM Designation: A923-06, Standard Test Methods for detecting Detrimental Intermetallic Phases in Duplex Austenitic/Ferritic Stainless Steel. [ Links ]
©The Southern African Institute of Mining and Metallurgy, 2012. SA ISSN2225-6253. This paper was first presented at the ZrTa2011 New Metals Development Network Conference, 12-14 October 2011, Mount Grace Country House & Spa, Magaliesburg.














