Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 spe Johannesburg Jul. 2012
TRANSACTION PAPER
Phase transformations and surface characterization of the platinum-chromium coated system
N. HaniefI; C.I. LangI; R. BucherII; M. TopicII
ICentre for Materials Engineering, Department of Mechanical Engineering, University of Cape Town
IIMaterials Research Department, iThemba Laboratory for Accelerator Based Sciences
SYNOPSIS
This research involves the investigation of phase transformations in the platinum-chromium coated system. Single-layer 0.1 ìçé platinum coatings were deposited via electron beam deposition on 99.98 percent pure chromium substrates. Specimens were subjected to systematic heat treatment in a vacuum furnace at 900°C for up to 20 hours. Phase formation and the changes in surface morphology were investigated by X-ray diffraction (XRD) and scanning electron microscopy (SEM). Both CrPt and Cr3Pt phases are formed during heat treatment for different times at 900°C. Significant changes in the morphology of this coated system were detected after heat treatment at 900°C for 20 hours.
Keywords: Platinum-chromium system, platinum coating, chromium substrate, phase formation , surface morphology.
Introduction
The platinum-chromium (Pt-Cr) system is used in many applications, including magneto-optics and micro-electronics. Platinum (Pt) can be used for ohmic contacts, and when alloyed with chromium (Cr), the reliability of this contact is greatly improved1. Pure chromium has a favourable strength-density ratio with excellent corrosion resistance. However, this metal has numerous problems in production, shaping, and joining2. Its excellent corrosion resistant properties and high melting temperature make it useful in functional coatings and for improving wear and corrosion resistance2. Coated systems offer the potential to control experimental parameters such that specific phases can be formed with desired mechanical properties3,4. The literature on platinum-chromium coated systems is limited, and thus further research should be conducted on this coated system.
A solid-state interfacial reaction occurs upon heat treatment, resulting in diffusion of Pt and Cr to form various phases. Diffusion in thin films differs from that in bulk systems due to differences in structure and grain boundary activation energy5. For diffusional phase transformations, thin films exhibit characteristics such as sequential formation of phases, which are not seen in bulk diffusion couples3,4. This can also bring about the formation of ordered intermetallic compounds, which can alter the mechanical properties of the material. This study aims to determine the sequence of phase formation and development of surface morphology for the platinum-chromium coated system, with a view to contributing to electronic device optimization. This work is also aimed at developing a better understanding of the thermodynamic and kinetic description of the formation of phases in this system.
The thermodynamic description of the Pt-Cr system predicts the Cr3Pt phase to form initially which is in accordance with the effective heat of formation (EHF) model described below. The Cr3Pt phase has a more negative heat of formation than the CrPt and Pt3Cr phases3. This is seen in Figures 1(a) and (b), which portray the equilibrium phase diagram and the effective heat of formation diagram respectively.
The effective heat of formation model3,4
Pretorius et al. 3,4 describes an efficient model that makes accurate predictions on phase formation. The EHF model is a thermodynamic model used to predict the first and sequential formation of phases. This model makes use of thermodynamic data such as a modified heat of formation (AH'), which is dependent on the effective concentrations (Equation [1]) of the elements reacting at the interface of the substrate and coating. The activation energy for solid-state interdiffusion is directly proportional to the melting point of the solid and thus determines the mobility of the atoms during diffusion3,4. The effective concentration is thus taken at the composition of the liquidus minimum as this is the point of highest mobility of atoms at the interface.

where ΔH0 is the heat of formation of the compound phase.
The rules for predicting phase formation in this model state that 'The first compound phase to form during metal-metal interaction is the phase with the most negative effective heat of formation at the concentration of the lowest temperature eutectic of the binary system' and 'the next phase to form at the growth interface is the next phase richer in the unreacted element'3-4. In Figure 1 (a) shows that two liquidus minima exist for the Pt-Cr system at approximately similar temperatures (1 500°C at ~13 at.% Pt and 1 530°C at ~24 at.% Pt) and thus the prediction of the first phase in this system is more uncertain3,4. Figure 1 (b) predicts the initial phase formation to be the Cr3Pt phase using the liquidus minimum at 13 at.% Pt and the next phase formed to be CrPt. However, due to the high affinity of chromium for oxygen, it could be expected that the effective concentration of this system is shifted to the Pt-rich side on the EHF diagram3,4. Thus the liquidus minimum at 24 at.% Pt would be used and the first phase would be predicted to be the CrPt phase3,4.
Experimental procedure
Pure platinum (99.99 percent) was used as a target material for the electron beam deposition of 0.1 ìçé platinum coatings on 99.98 percent pure chromium substrates. Cast chromium pieces were cut, ground, and polished to a mirror finish using a colloidal silica suspension. Substrates were then cleaned in different solutions such as methanol, ethanol, acetone, distilled water, and hydrofluoric acid in an ultrasonic bath prior to deposition. Platinum was then deposited with the use of an evaporation system and an electron beam source. After deposition, the specimens were subjected to systematic heat treatments in a vacuum furnace at a high temperature of 900°C.
Phase formation and the changes in surface morphology were investigated by X-ray diffraction (XRD) and scanning electron microscopy (SEM). These were used as complementary techniques in this study. A BRUKER ADVANCE-8 diffractometer was used with CuKa radiation at 40 kV and 40 mA for XRD, and a NOVA NANOSEM 230 at 20 kV and a magnification of 10 000x was used for microscopy images and energy dispersive X-ray spectroscopy (EDX).
Results and discussion
X-ray diffraction
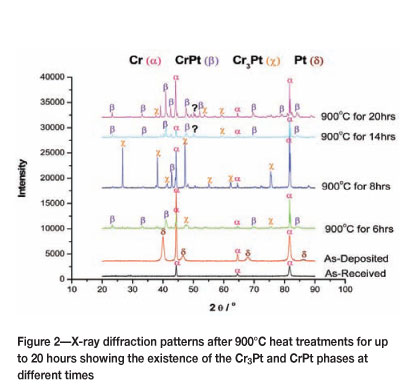
In Figure 2 the XRD patterns of the platinum-coated chromium substrates heat treated at 900°C for different times are displayed. As-received plots show Cr peaks, whereas as-deposited plots show both Cr and Pt peaks. The CrPt and Cr3Pt phases are observed at all times taken into consideration at this temperature; however, a significant difference is noted at the 8 hour time interval. The volume fraction of the Cr3Pt phase is observed to be the highest at this time, and longer annealing periods caused a decrease in the Cr3Pt volume fraction. It is also observed that after long annealing periods the volume fraction of the CrPt phase is increased. This is particularly pronounced after annealing for 20 hours. It is clear that the volume fractions of these phases change with increasing annealing time. Since only a few time intervals were considered, a definite phase formation sequence cannot be predicted according to the EHF model. It should also be noted that there are peaks that could not be identified due to the lack of information in the XRD database. Further studies of the phases in this system need to be conducted in order to provide an accurate prediction of phase formation sequence.
Electron microscopy
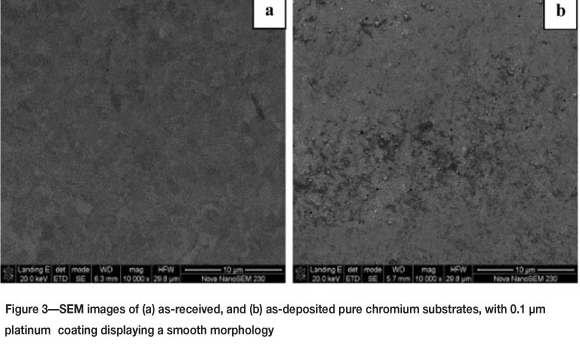
The surface morphology is smooth before and after electron beam deposition. Figure 3 shows SEM images of (a) as-received and (b) as-deposited specimens. After heat treatment at high temperatures, a solid state interfacial reaction occurs, resulting in diffusion between the platinum coating and chromium substrate. Figures 4 (a) to (d) show SEM images of platinum-coated chromium substrates after heat treatment at 900°C from 6 hours up to 20 hours. Vast differences in morphology are evident with respect to time, with a general increase in coating roughness. Figures 4 (a) to (c) display a granular morphology that progressively increases in size with time. In Figure 4 (d) chromium-rich needle-like crystals are formed on the surface of the coating, with EDX analysis showing a composition of 100 at.% Cr in these crystal regions. The crystals protrude from the surface in different orientations after annealing at an approximate angle of 45°.
Summary and conclusion
Phase transformations in the Pt-Cr coated system occur as per the platinum-chromium equilibrium phase diagram and include the Cr3Pt and CrPt phases. The sequence of phase formation at the interface between the Cr and Pt at 900°C cannot, however, be determined in this study due to the few time intervals being considered. The coating morphology is significantly affected by heat treatment, where large needlelike crystals are formed on top of the coating surface. These results have not been previously observed and thus serve as a basis for further study on this system. This would entail the effects of this morphology on the mechanical properties of Pt-Cr coatings and the continued study of phase formation.
Acknowledgements
The authors acknowledge the financial support of Mintek and the National Research Foundation, the use of the EMU of the University of Cape Town and the XRD facilities at iThemba LABS.
References
1. Danyluk, S., McGuire, G.E., Koliwald, K.M., and Yang, M.G. Diffuion studies in thinfflms using uger electron spectroscopy. Thin Solid Films, vol. 25, 1975. pp. 483-489. [ Links ]
2. Holzwarth, U. and Stamm, H. mechanical and thermomechanical properties of commercially pure Cr and Cr alloys. Journal of Nuclear Materials, vol. 300, 2002. pp. 161-177. [ Links ]
3. Pretorius, R., Marais, T.K., and Theron, C.C. Thin film compound and phase formation sequence: an effective heat of formation model. Materials Science and Engineering, vol. 10, 1993. pp. 1-83. [ Links ]
4. Theron, C.C., Ndwandwe, O.M., Lombaard, J.C., and Pretorius, R. First phase formation at interfaces: comparison between Walser-Bené and effective heat formation model. Materials Chemistry and Physics, vol. 46, 1996. pp. 238-247. [ Links ]
5. Ostrovsky, A.S. and Bokstein, B.S. Grain boundary diffusion in tin films under stress fields. Applied Surface Science, vol. 175, 2001. pp. 312-318. [ Links ]
6. Pinto, L.M.C., Silva, E.R., Caram, R., Tremiliosi-Filho, G., and Ângelo, A.C.D. Preparation and characterisation of ordered intermetallic platinum phases for electrolytic applications. IntermetalUcs, vol. 16, 2008. pp. 246-254. [ Links ]
7. Zhang, C., Zhu, J., Bengtson, A., Morgan, D., Zhang, F., Yang, Y., and Chang, Y.A Thermodynamic modelling of the Cr-Pt binary system using cluster/site approximation coupling with first-principles energetics calculation. Acta Materiala, vol. 56, 2008. pp. 5796-5803. [ Links ]
©The Southern African Institute of Mining and Metallurgy, 2012. SA ISSN2225-6253. This paper was first presented at the ZrTa2011 New Metals Development Network Conference, 12-14 October 2011, Mount Grace Country House & Spa, Magaliesburg.














