Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 no.12 Johannesburg Jan. 2012
Potential for bioleaching copper sulphide rougher concentrates of Nchanga Mine, Chingola, Zambia
J. ManchisiI; G.S. HansfordI; P. GaylardI; S. SimukangaII; R.L. NyirendaII; and A. SichalweIII
IBioprocess Engineering Research Unit, Department of Chemical Engineering, University of Cape Town, Rondebosch, South Africa
IIDepartment of Metallurgy and Mineral Processing, University of Zambia, Lusaka, Zambia
IIIKonkola Copper Mines Plc, Chingola, Zambia
SYNOPSIS
Laboratory investigations were conducted to establish the feasibility of bioleaching a mixed copper oxide/sulphide rougher concentrate from Nchanga Mine on the Zambian Copperbelt. The objective was to determine the kinetics and extent of copper extraction for this material. Batch experiments were conducted under different solution conditions in stirred tank bioreactors. The progress of (bio)leaching was monitored through measurements of soluble ferrous and ferric iron, copper, pH, and redox potentials, while bacterial activity was monitored online through O2 and CO2 gas utilization rates.
About 20 per cent copper was solubilized within 2 hours in all cases of non-oxidative (abiotic), oxidative (abiotic), and bioleaching experiments. This was attributed to the dissolution of mainly copper oxides. Subsequently, bioleaching experiments resulted in an overall copper extraction of 93 per cent, with up to 8 g.L-1 copper after six leaching days, compared to 58 per cent copper extraction in the abiotic oxidative acid leaching experiments. However, there was little effect of time (i.e. poor dissolution kinetics) on copper recovery for abiotic non-oxidative acid leaching of the material.
Hence, the rate of sulphide leaching increased due to the activity of bacteria. Thus, the material is potentially bioleachable under mesophilic conditions. However, more exhaustive test work needs to be conducted to establish the effect of bioleaching variables and heat requirement.
Keywords: bioleaching, bioreactor, copper sulphide ore, recovery, kinetics.
Introduction
Bioleaching of low-grade, mixed copper oxide/sulphide ores in bioheaps is now an established technology1 However, bioleaching efficiency and kinetics vary significantly for ores from different localities due to different mineralogical compositions, bacterial species, solution conditions, and the leaching system employed.
Dreisinger2 and Miller,3 reported that bioleaching may be a cheaper alternative to other traditional metal extraction techniques such as smelting and therefore more suited to treating marginal (or low-grade) ores in cases where high-grade mineral reserves have been depleted. It was therefore necessary to investigate the amenability of Nchanga copper sulphide material to bioleaching. This preliminary leaching kinetics data may be useful for the design, and possible development and operation, of tank or heap bioleaching operations.
The purpose of this study was to determine the kinetics of copper extraction, and the extent of mineral dissolution and microbial activity, on copper dissolution using a mixed copper oxide/sulphide rougher concentrate from Nchanga Mine as being representative of the Zambian Copperbelt, using a mixed culture of iron- and sulphur-oxidizing mesophilic bacteria.
Copper bioleaching
A literature survey on copper bioleaching has indicated that secondary sulphide minerals of copper (such as chalcocite, covellite, and bornite) have been demonstrated to show satisfactory bioleach kinetics with mesophilic bacteria4, with several bioheap operations worldwide extracting copper under these conditions1. However, chalcopyrite has been reported to be very difficult to leach under mesophilic conditions. Attempts to improve chalcopyrite bioleaching now involve the application of controlled solution redox potentials, use of silver as a catalyst, and the application of thermophilic bioleaching to quickly and completely leach chalcopyrite5,6. A recent economic but non-bioleach hydrometallurgical system for the treatment of primary copper concentrates consisting predominantly of chalcopyrite is the GalvanoxTM Process7. In this process, galvanic activity between pyrite and chalcopyrite promotes rapid and complete chalcopyrite oxidation to give over 98 per cent copper recovery within 4 hours at 80°C. A brief summary of the electrochemistry of the process is given in Equations [1-3].
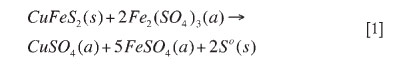
Reaction [1] can be separated into anodic and cathodic half-cell reactions. The anodic half-cell reaction is:
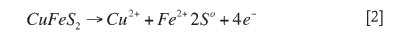
and the cathodic half-cell reaction is:

Chalcocite is an important secondary sulphide of copper that is reported to leach through a two-stage mechanism. It is rapidly attacked by ferric iron during the first stage to give a CuS intermediate product, while the second stage proceeds more slowly. About half (50 per cent) of the copper in Cu2S is leached quickly, though a complete dissolution requires much longer times8-10. Under mesophilic conditions, high rates of copper extraction from chalcocite have been reported for low pulp densities of typically less than 5% w/v solids, pH 1.3-2.3, 25-40°C, and quite low ferric iron concentration4,11,12.
It is well established that the first-stage leaching of chalcocite occurs faster than the microbial ferrous iron oxidation rate and produces non-stoichiometric pseudo covellite, which reacts further rather slowly in the second stage to produce S0, Cu2+, and Fe2+ ions as detailed below8,10,13. At the same time, Fe2+ and S0 are microbially oxidized to regenerate Fe3+ and produce more acid respectively14-17.
First-stage leaching of chalcocite:
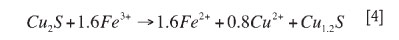
Second stage leaching of chalcocite or pseudo covellite by ferric iron is given by
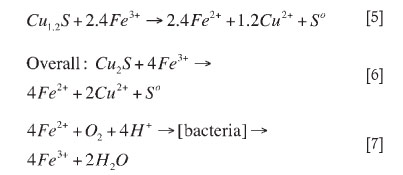

Microbial growth kinetics and O2 and CO2 gas uptake
According to Roels18 the biomass (CH1.8O0.5N0.2) uses CO2 and NH4+ as sources of carbon and nitrogen respectively for cell growth and maintenance. So the rate of bacterial growth, rx (C-mol.L-1) is equal to the rate of carbon dioxide utilization, rCO2.
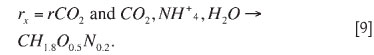
The biomass concentration in a batch at time t is calculated from integration of the measured carbon dioxide rate14 through a computer program called 'Off-gas analyser' that continuously monitors and logs CO2 utilization rates by microbes.
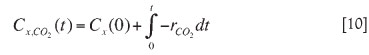
Materials and methods
Mineral characterization
The composite mineral samples were copper sulphide rougher concentrates provided by Nchanga Mine, Zambia. These samples were characterized in terms of particle size distribution (PSD) using a Malvern Mastersizer, chemical analysis was by atomic absorption spectrophotometer (AAS), while the mineralogical composition, distribution, and liberation characteristics were investigated using reflected light microscopy.
Chalcocite, at 15.27 weight per cent (wt%), was the major sulphide, followed by oxides of malachite (1.24 wt%), cuprite (1.67 wt%), and cupriferous micas (13.0 wt%). Chalcopyrite (1.53 wt%) also occurred in significant amounts. From elemental analysis the head grades were 16 per cent total copper, 7.01 per cent iron, and 9.51 per cent sulphur. Over 70 per cent of the particles were less than 38 μm, with d50 being 16 μm The liberation characteristics were fairly good except for chalcopyrite, where 17 per cent remained locked in the gangue minerals.
Experimental procedure
Several studies involving copper sulphides have been conducted in shake flask experiments where cell/microbial growth rates are often measured manually using such techniques as cell counts, protein analysis etc. Due to the relatively slow kinetics associated with shake flask experiments, low accuracy, and longer time requirements in the more common cell count technique, this study employed a stirred tank leaching method using off-gas analysis to enumerate microbial cells following the methods of Boon14 and Breed19.
In fact, reported bioleaching studies involving copper sulphide ores and/or minerals coupled to the off-gas for analysis of microbial growth are rather few, therefore it was necessary to conduct this study using the online off-gas technique as a faster alternative tool to enumerate microbial cells. Similar solution conditions to those of published studies involving shake flask experiments were therefore applied in this study to investigate the extent of copper extraction in stirred tank bioreactors.
Determination of acid consumption
Since the concentrate contained mixed copper oxide/sulphide mineralization, it was necessary to determine acid requirement and acid soluble copper as a test prior to bioleaching. A batch stirred tank reactor was used with solids concentration of 5% w/v at pH 1.5, for which pH adjustments were done by the addition of sulphuric acid through a burette.
Batch culture, nutrients, and shake flask experiments
Erlenmeyer flasks were used for growth and adaptation of bacteria in rotary shakers using a modified 9k (iron-free) basal salts growth medium with the ore as substrate at 5% w/v. Both the ore and glassware were sterilized separately by autoclaving at 121°C for 20 minutes. A mixed culture of iron- and sulphur-oxidizing, chemolithoautotrophic mesophilic bacteria, obtained from a University of Cape Town (UCT) laboratory stock culture, was used in the study.
The bacterial culture was grown in a modified 9k medium (i.e., mineral salt solution) with: (NH4)2SO4 (3.0 g.L-1), KCl (0.1 g.L-1), K2HSO4 (0.5 g.L-1),MgSO4.7H2O (0.5 g.L-1), and Ca(NO3)2.4H2O (0.013 g.L-1). The pH was adjusted to 2.0. The flasks were then inoculated with the bacterial culture at 10% v/v using aseptic techniques, and incubated at 35°C with a shaker speed of 170 r.min-1 The microbial growth rates were monitored frequently by cell counts and sub-culturing until growth of about 108-109 cells per millilitre was observed.
Batch chemical and microbial leaching experiments
These experiments were performed in a baffled, stirred, and aerated bioreactor (Figure 1) that was thermostated by an external water bath. O2 and CO2 contents in the dry off-gas and reference air were measured and monitored online using a data acquisition program.
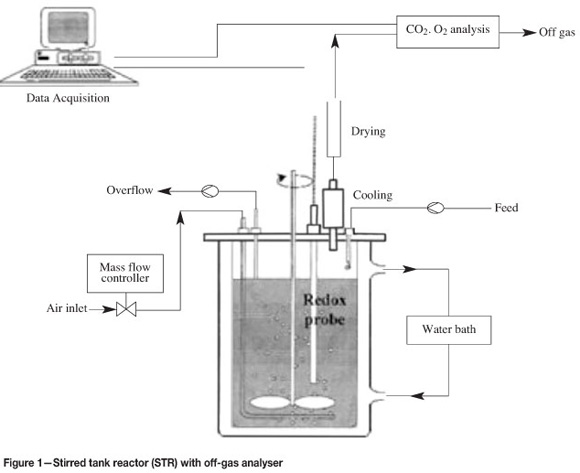
The difference between reference and off-gas concentration was the microbial gas utilization rates, from which biomass concentration (C mole/litre) was calculated. Both microbial and chemical leaching experiments were conducted in the same reactor type and solution conditions, except that for the chemical leaching experiments the bacterial inoculum was replaced with an equivalent volume of sterile growth medium to make up 1 litre working volume.
Fifty (50) grams of mineral sample was added to the nutrient medium and the pH adjusted to the required value using sulphuric acid. The reactor was then inoculated with 100 ml of bacterial culture per 900 ml solution. When the pH was higher than the set value it was adjusted to this value by acid addition. If the pH was lower than required, a freely changing value was allowed to determine the acid-producing capacity of the bioleach system. If a constant pH was desired, it was manually controlled by the addition of either acid or base.
Daily 10 ml slurry samples were taken, filtered, and analysed for soluble species. The residues were digested to determine unleached copper and iron. Progress of bioleaching was monitored through measurements of [Fe2+], [Fe3+], [Cu2+], pH, and redox potential, while bacterial activity was monitored by CO2 and O2 utilization rates.
The redox potential was measured with a gel-filled Pt-Ag/AgCl combination redox probe. The pH values were measured by a combination probe using a Model 744 Metrohm pH meter filled with 3.0 M KCl. Copper and total iron concentrations were measured by AAS, while ferrous iron (Fe2+) was determined by spectrophotometry using 1-10 phenanthroline (C12H8N2H2O) as an indicator and ammonium acetate (NH4C2H3O2) as a buffer solution. Ferrous (Fe2+) iron was also determined by actual calculations from values of measured redox potentials, and total iron concentration using the Nernst equation20 as shown in Equation [11]. The ferric (Fe3+) iron concentration was taken as the difference between total and ferrous iron contents (Fetotal = Fe2+ + Fe3+).
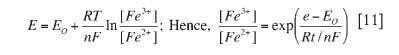
where E = potential of the solution (V), Eo = standard potential of Fe3+/Fe2+ couple (V), R = universal gas constant (J.K-1moL-1), T = absolute temperature (K), n = number of electrons transferred, F = Faraday's constant (kj.v-1 per equivalent), [Fe3+], and [Fe2+] = molar concentration of ferric and ferrous ions respectively (mol.L-1)
Thus, the Nernst equation relates the solution potential to the free ferric and ferrous iron activities with ideal values of Eo and RT/nF. Hence, the redox probe was calibrated20 at the applied experimental conditions in order to obtain these coefficients, Eo and RT/nF. Calibration Equation [12] at 35°C and pH 2.0 was:
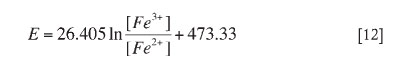
Hence, at each redox potential value and total iron concentration, values of ferrous and ferric iron concentrations were calculated by the simplified set of Equations [13]:
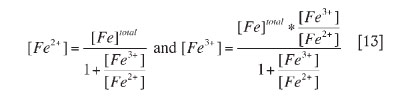
The calculations of percentage copper extraction were based on both soluble copper and leach residue assays. The use of leach residue assays was found to be more consistent and reliable. This procedure was adopted and used in the calculations (Equation [14]). Total iron dissolution was calculated similarly.
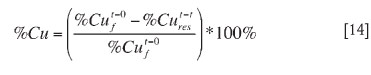
where % 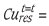 per cent copper in leached residue at any time t and %
per cent copper in leached residue at any time t and % 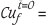 per cent copper in starting (feed) sample at time t = 0.
per cent copper in starting (feed) sample at time t = 0.
Abiotic oxidative and non-oxidative acid leaching experiments
In line with the previous section, acid leach tests were conducted to determine acid consumption by oxide copper and gangue minerals. The extent of dissolution of acid-soluble copper was assessed through oxidative (with air) and non-oxidative (without air) leaching. The objective was to determine the extent of copper dissolution in the absence of bacteria.
Microbial leaching
The bioleaching experiments were conducted on both fresh rougher concentrate feeds and leach residues from non-oxidative acid leach experiments. Details of the procedure are given in the previous sections.
Results and discussion
Acid consumption
It is expected that copper oxides, carbonates, silicates, and other alkaline gangue minerals rapidly consume acid to break down to different solution products21. Therefore, preliminary acid consumption tests were designed to approximate the quantitative acid requirement of rougher concentrates at the desired pH, and also to infer the extent of dissolution of acid-soluble copper and slow-leaching oxides and sulphides.
In the initially observed pH profiles, the acidity of the leaching system decreased, and this was attributed to chemical breakdown of both acid-soluble copper mineral phases and gangue (waste), as noted by Jansen and Taylor21. The bioleaching tests exhibited higher acid consumption rates, resulting in higher copper extractions than the abiotic oxidative acid leaching experiments. This may be due to additional acid-consuming reactions in bioleaching, such as microbial ferrous iron oxidation (Equation [7]). Acid demand was 150-300 kg and 150-240 kg per ton of ore for bioleaching and abiotic oxidative acid leaching tests respectively. Similar rates of acid consumption were reported for copper oxide leaching22.
Oxidative, non-oxidative, and microbial leaching
In Figure 2, the abiotic non-oxidative acid leaching tests solubilized about 20 per cent copper after 50 hours, compared to 45 per cent copper dissolution in the oxidative leaching tests using air. The dissolution profile starts with complete acid dissolution of CuO and copper carbonates and dissolution of about half of the Cu2O 22,23. Introduction of air (O2) probably leads to the complete dissolution of cuprite (which requires oxygen) and partial oxidation of chalcocite24 to an overall 45 per cent copper extraction, since most other copper sulphides are relatively unaffected under these conditions25. The chemical leaching reaction mechanisms are given in Equations [15-21].

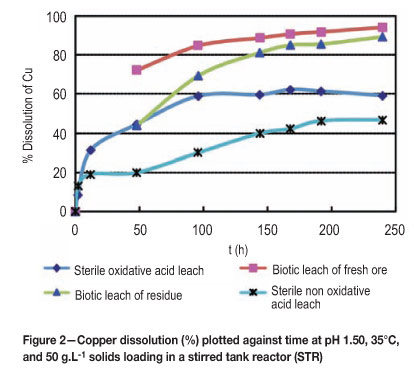
Cuprite (Cu2O) theoretically solubilizes only half of the copper (Equation [19]) while the other half is converted into metallic copper which subsequently undergoes dissolution in the presence of oxygen (Equation [20]).
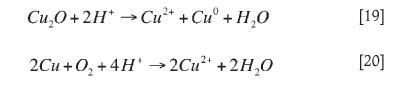
Reaction [20] is a summation of two half-cell reactions:
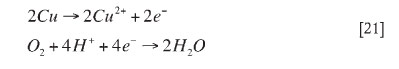
In the bioleach system over 93 per cent of the copper was solubilized in 6 days relative to 58 per cent copper extraction in the abiotic oxidative acid leach (Figure 2). The low leach efficiency in acid leach tests was attributed to limited acid dissolution of copper sulphides. Thus, in the presence of bacteria the rate of sulphide oxidation is enhanced. The production of ferric iron from microbial ferrous iron oxidation (Equation [7]) leads to the ferric leaching of copper, pyrite and other sulphides to varying degrees, as given for Cu2S (Equations [4-8]), pyrite, and other sulphides (Equations [22-27])17,26.
Secondary copper sulphides are nearly completely bioleached with mesophilic bacteria1,4,8,27, resulting in the observed high copper extraction rates. The residual copper might be attributed to chalcopyrite because this mineral has been reported to be very difficult (refractory) to leach with mesophiles4,26,28,29.
Chemical ferric oxidation of pyrite:
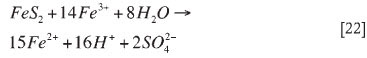
Bacterial ferrous iron oxidation:
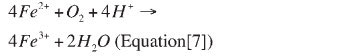
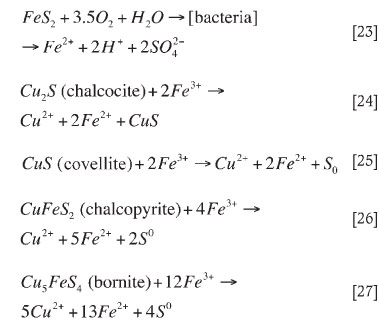
Dew et alA reported the preferential order of mineral bioleaching with mesophile bacterial culture as Cu2S > Cu5FeS4 > CuS> FeS> ... > CuFeS2.
The bioleaching of a pre-treated rougher concentrate (residue) from the non-oxidative acid leach experiment was observed to initially proceed slowly relative to the untreated ore, though eventually giving over 93 per cent copper extraction in both cases. This is because the leach residue probably did not contain acid-soluble copper oxides. Furthermore, the experimental copper dissolution data compared well with published laboratory bioleaching studies4,8,10,11. The consequence of these trends is that bioleaching of untreated ore may be preferable because of the faster oxidation kinetics.
Variation of redox potential and ferrous/ferric iron
The initially low redox potential values noted during the bacterial lag phase were probably due to the relatively fast reduction of ferric iron by chalcocite during the first stage of leaching. This was followed by onset of bacterial oxidation of ferrous iron, as reflected in the rapid increase of redox potential (Figures 3 and 4). However, the rate of mineral ferric consumption at the second stage is now much lower and the solution redox potential attains high values. Redox potentials are reflected by changes in the Fe3+/Fe2+ ratios of dissolved iron in the leach slurry reaching 690 mV (vs. Ag/AgCl) (Figure 3). The bacterial ferrous oxidation reaction is probably limited by a low ferrous iron concentration (Figures 3 and 4).
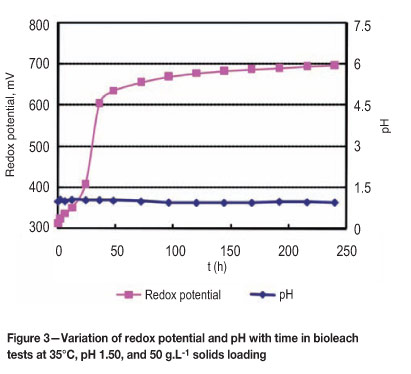
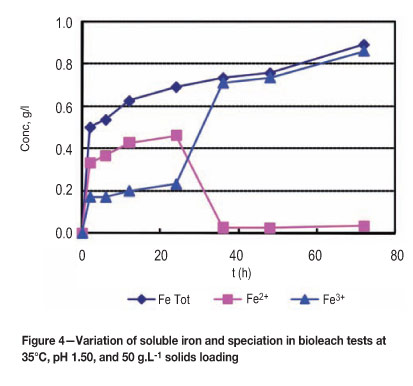
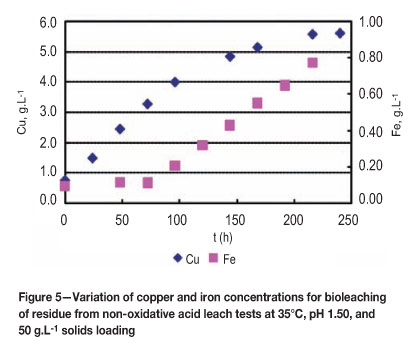
Also, the dissolution of pyrite (Equations [7], [22], and [23]) was significant only at high redox potentials from observed measurements of total soluble iron (Figure 5). Thus, it is likely that pyrite dissolution started after about 75 hours when the solution redox potential was above 550 mV (vs. Ag/AgCl) (Figure 5). It has been reported that pyrite dissolution occurs at high redox potential after considerable dissolution of secondary copper sulphides and to some extent chalcopyrite30.
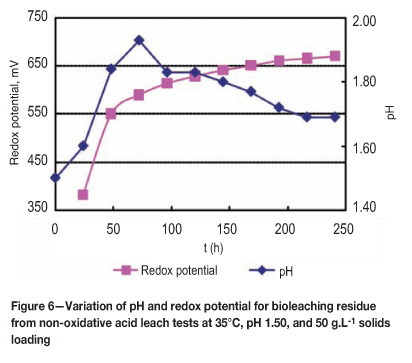
Sulphur-oxidizing bacteria probably oxidized sulphur and/or reduced sulphur compounds to sulphuric acid (Equation [8]) since the pH was observed to decrease, indicating a bacterial activity after 3 days (Figure 6). It appears therefore that ferrous iron and sulphur compounds (and not chalcocite) are the substrates for bacteria. They regenerate ferric iron that subsequently oxidizes chalcocite and other sulphides. These results are consistent with literature data, confirming and recognizing the fact that the presence of bacteria increases the rate of leaching. The role of bacteria therefore is to provide acid and the ferric iron oxidant (through sulphur and ferrous iron oxidation) required for dissolution of copper oxides/carbonates and sulphides.
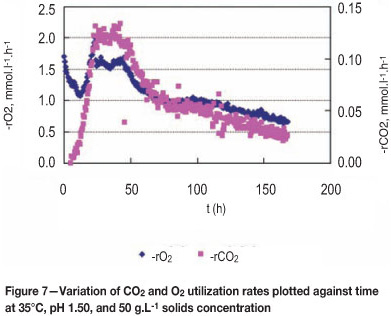
In Figures 7 and 8, the growth and activity of bacteria were dependent on ferrous iron concentration. Bacterial O2 and CO2 consumption rates were rapid and reached 2.0 mmol. L-1.h-1 and 0.131 mmol.L-1.h-1 respectively after 24 hours. The concentration of bacteria was a maximum in the last phase at about 10.0 mmole carbon per litre, after which there was negligible rate of growth other than probably in maintenance terms.
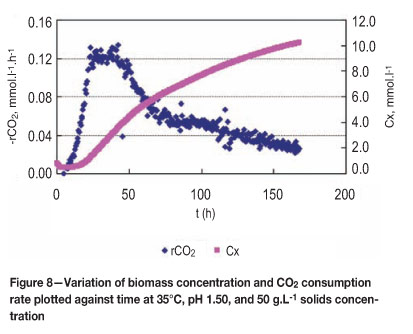
Conclusions
The leaching profile of this material progressed in a three phase sequence. In phase one, 20 per cent copper was abiotically leached in dilute acid without air (oxygen), corresponding to the chemical (acid) dissolution of copper oxides/carbonates. The second phase resulted in 40-60 per cent copper being abiotically and chemically extracted in the presence of air. The last phase involving bioleaching of largely copper sulphides resulted in over 93 per cent copper recovery, with up to 8.0 g.L-1 Cu after six leaching days. Hence, the material responded positively to bioleaching under mesophilic conditions as evidenced by high percentage copper dissolution and satisfactory microbial activity of up to 10.0 mmol carbon per litre bacterial population at the stationary phase. Thus, off-gas analysis is useful for calculating microbial growth rates and other kinetic parameters even in a multi-sulphide leaching system, as it has been done for single mineral substrates like pyrite and chalcopyrite14.
However, more exhaustive test work needs to be conducted to establish the effect of bioleaching variables and heat requirement. Further investigations in columns are needed to ascertain the leachability and scale-up of the present experimental data to a possible heap bioleaching operation. It is also proposed that a rigorous mathematical treatment of degree of reduction balances must be applied to a multi-substrate system such as this one in order to relate the rate of copper dissolution to bacterial O2 and CO2 consumption rates, as has been done for single (ferrous iron, sulphur, etc.) substrates by Boon14. One limitation in this study was the inability to determine the qualitative and quantitative mineralogical composition of the bioleach residues to fully interpret the bioleaching data.
Acknowledgement
The authors would like to thank University Science, Humanities and Engineering Partnerships in Africa (USHEPiA) in collaboration with the University of Cape Town (UCT), the University of Zambia (UNZA), and Konkola Copper Mines (KCM) plc for funding this research project.
References
1. WATLING, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides - a review. Hydrometallurgy, vol. 84, no. 2, 2006. pp. 81-108. [ Links ]
2. DREISINGER, D. Copper leaching from primary sulphides: options for biological and chemical extraction of copper. Hydrometallurgy, vol. 83, 2006. pp. 10-20. [ Links ]
3. MILLER, P.C. The design and operating practice of bacterial oxidation plant using moderate thermophiles (the BacTech Process). Biomining: Theory, Microbes and Industrial Processes. Rawlings, D.E. (ed.). Springer, Berlin and Landes Bioscience, Austin, TX, 1997. pp. 81-102. [ Links ]
4. DEW, D.W., VAN BUUREN, C., MCEWAN, K., and BOWLER C. Bioleaching of base metal sulphide concentrates. A comparison of mesophile and thermophile cultures. Biohydrometallurgy and the Environment toward the Mining of the 21st Century. Proceedings of International Biohydrometallurgy Symposium, IBS '99, El Escorial, Spain. Amils, R and A. Ballester (eds), Elsevier, Amsterdam, 1999. pp. 229-238. [ Links ]
5. GERICKE, M. and PINCHES, A. Bioleaching of copper sulphide concentrates using extreme thermophilic bacteria. Minerals Engineering, vol. 12, no. 8, 1997. pp. 893-904. [ Links ]
6. MOUSAVI, S.M., YAGHAMAEI, S.M. VOSSOUGHI, M., JAFARI, A., and HOSEINI, S.A. Comparison of bioleaching ability of two native mesophilic and thermophilic bacteria on copper recovery from chalcopyrite concentrate in airlift bioreactors. Hydrometallurgy, vol. 80, 2005. pp. 139-144. [ Links ]
7. Dixon, D.G., Mayne, D.D., and Baxter, K.G. GalvanoxTM - A novel galvan-ically-assisted atmospheric leaching technology for copper concentrates. Canadian Metallurgical Quarterly, vol. 47, no. 3, 2008. pp. 327-336. [ Links ]
8. DUTRIZAC, J.E. and MACDONALD, R.J.C. Ferric ion as a leaching medium. Minerals Science and Engineering, vol. 6, no. 2, 1974. pp. 59-100. [ Links ]
9. ROSSI, G. Biohydrometallurgy. McGraw-Hill, Hamburg, Germany, 1990. [ Links ]
10. BOLORUNDURO, S.A. Kinetics of leaching of chalcocite in acid ferric sulphate media: chemical and bacterial leaching. MSc Thesis, University of British Columbia, Canada, 1990. [ Links ]
11. RIVERA-SANTILLAN, R.E., BALLESTER PEREZ, A., BLAZQUEZ IZQUIERDO, M.L., and GONZALEZ, F. Bioleaching of a copper sulphide flotation concentrate using mesophilic and thermophilic micoroorganisms. Biohydrometallurgy and the Environmental toward the Mining of the 21st Century, Proceedings of Biohydrometallurgy Symposium, IBS '99, El Escorial, Spain, June 1999. Amils, R. and Ballester, A. (eds.). Elsevier, Amsterdam, 1999. pp. 149-158. [ Links ]
12. SAKAGUCHI, H., TORMA, A.E., and SILVER, M. Microbiological oxidation of synthetic chalcocite and covellite by Thiobacillus ferrooxidans. Applied Environmental Microbiology, vol. 31, no. 1, 1976. pp. 7-10. [ Links ]
13. PETERSEN, J. and DIXON, D.G. The dynamics of chalcocite heap bioleaching. Hydrometallurgy 2003. Proceedings of the 5th International Symposium Honoring Professor Ian M. Ritchie. vol. 1. Leaching and solution purification. Young, C., Alfantazi, A., Anderson, C., James, A., Dreisinger, D., and Harris, B. (eds.). TMS, Warrendale, PA, 2003. pp. 351-364. [ Links ]
14. BOON, M. Theoretical and experimental methods in modelling bio oxidation kinetics of sulphide minerals. PhD thesis, Technical University Delft, The Netherlands, 1996. [ Links ]
15. SAND, W., GEHRKE, T., HALLMAN, R., and SCHIPPERS, A. Sulphur chemistry, biofilm, and the (in)direct attack mechanism-critical evaluation of bacterial leaching. Applied Microbiology and Biotechnology. vol. 43, 1995. pp. 961-966. [ Links ]
16. SAND, W., GEHRKE, T., JOZSA, P.G., and SCHIPPERS, A. Biochemistry of bacterial leaching - direct vs. indirect bioleaching, Hydrometallurgy, vol. 59, 2001. pp. 159-175. [ Links ]
17. SCHIPPERS, A. and SAND, W. Bacterial leaching of metal sulphides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulphur, Applied Environmental Microbiology, vol. 65, 1999. pp. 319-321. [ Links ]
18. ROELS, J.A. Energetics and Kinetics in Biotechnology. Amsterdam, Elsevier Biomedical Press, 1983. [ Links ]
19. BREED, A.W. Studies on the mechanism and kinetics of bioleaching with special reference to the bioleaching of refractory gold-bearing arsenopyrite/pyrite concentrates. Ph.D thesis, University of Cape Town, South Africa, 2000. [ Links ]
20. NEMATI, M. and WEBB, C. A kinetic model for biological oxidation of ferrous iron by Thiobacillus ferrooxidans. Biotechnolology and Bioengineering, vol. 53, no. 5, 1996. pp. 478-486. [ Links ]
21. JANSEN, M. and TAYLOR, A. Overview of gangue Mineralogy issues in oxide copper heap leaching. International Project Development Services Pty Ltd, Mona Vale, New South Wales, Australia, 1998. [ Links ]
22. BUSTOS, S., CASAS, J.M., and GONZALEZ, C. Acid requirements in bacterial heap leaching of copper sulphide ores. Hydro-sulphides, 2004, pp. 187-196. [ Links ]
23. NYIRENDA, R.L. Hydrometallurgy I and II, Department of Metallurgy and Mineral Processing, University of zambia, Lusaka, 2000, [ Links ].
24. OLSON, G.J., BRIERLEY, J.A., and BRIERLEY, C.L. Bioleaching review part B: Progress in bioleaching: applications of microbial processes by the minerals industries. Applied Microbiology and Biotechnology, 2003. pp. 249-257. [ Links ]
25. DRESHER, W.H. (2004). Producing copper nature's way: bioleaching. Copper Development Association. http://www.copper.org/publications/newsletters/innovations/2004/05/producing_ copper_natures_way_ bioleac hing.html. [ Links ]
26. MURR, L.E. Theory and practice of copper sulphide leaching in dumps and in-situ. Minerals Science and Engineering, vol. 12, no. 3, 1980. pp.121-189. [ Links ]
27. BRIERLEY, J.A. and BRIERLEY, C.L. Present and future commercial applications of biohydrometallurgy. Hydrometallurgy, vol. 59, 2001. pp. 233-239. [ Links ]
28. HACKL, R.P., DREISINGER, D.B., PETERS, E., and KING, J.A. Passivation of chalcopyrite during oxidative leaching in sulphate media. Hydrometallurgy, vol. 39, 1995. pp. 25-48. [ Links ]
29. SCHNELL, H.A. Bioleaching of copper. Biomining: Theory, Microbes and Industrial Processes. Rawlings, D.E. (ed.), Springer, Berlin and Landes Bioscience, Austin, TX, 1997. pp. 21-43. [ Links ]
30. MAY, N. The ferric leaching of pyrite. MSc thesis, University of Cape Town, South Africa, 1997. [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2012. ISSN2225-6253. Paper received Jun. 2010; revised paper received Nov. 2012.














