Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 n.12 Johannesburg Jan. 2012
SART for copper control in cyanide heap leaching
M. Stewart; D. Kappes
Kappes, Cassiday & Associates in the United-States
SYNOPSIS
Copper cyanide is a common component of cyanide-treatable precious metal ores. The copper concentration in production heaps can be predicted from laboratory column tests, but the exact correlation is not necessarily intuitive. Generally, heap leach operators like to keep copper concentrations in solution below 300-500 ppm and may note problems with gold recovery and cyanide consumption when copper concentrations exceed this amount. There are several methods of copper removal from cyanide solutions including ion exchange; direct electrowinning; acidification, volatilization, and recovery (AVR); and sulphide precipitation such as the sulphidization, acidification, recycling, and thickening (SART) process. SART involves acidification with addition of soluble sulphide, separation of the resulting copper sulphide precipitate, and addition of lime to re-establish alkalinity prior to returning the solution to the leaching process, recovering both copper and cyanide as valuable products. In principle SART is very simple. Yet some SART plants that have been built may have been unnecessarily complex. This paper explores the basics of SART and makes the case for a simple plant design as applied to the heap leaching circuit.
Keywords: SART, sulphidization, copper removal, copper cyanide, cyanide recovery, heap leaching.
Introduction
Many copper-laden precious metal deposits are, and have been historically, dismissed as economically and technically unattractive, with operators instead focusing on heap leach projects that do not require control of copper in the leaching process. However, with the decreasing number of such ideal projects, coupled with high metals prices in recent years, it is now more common to consider these copper-laden deposits. There are now several operations that have installed systems to control copper levels in the leaching process. The most common of these is the sulphidization, acidification, recycling, and thickening (SART) process. Without SART or some other system of copper removal, the copper content in field leach solutions would build up to levels that can create a variety of technical and economic complications.
Problems with copper in precious metal ores
The presence of cyanide-leachable copper in a large enough amount in a gold-bearing ore can be significantly detrimental to the economics of a gold project for several reasons:
- Copper, when dissolved with adequate free cyanide at the typical heap-leach or mill operating pH of 10-11, predominantly forms the Cu(CN)32- complex, in which copper will bind at least 2.3 kg of sodium cyanide for every kilogram of Cu leached. The reduction of free cyanide reduces the gold leaching rate and represents a significant consumption and inventory of cyanide in the leach circuit. The strategy of using a very low level of free cyanide may help alleviate this problem and result in the selective leaching of gold from some ores. However, if free cyanide is low enough or non-existent, the Cu(CN)2- complex or insoluble CuCN may form, and gold may not leach at all
- Copper affects accurate analysis of free or gold-leachable cyanide, complicating accurate cyanide dosing and cyanide control
- Copper competes with gold for adsorption on activated carbon in the normal adsorption/stripping circuit, particularly at low cyanide concentrations relative to copper, for example at CN/Cu ratios of <4 (Fleming and Nicol, 1984; Dai and Breuer, 2010). This can effectively reduce the gold loading capacity of the carbon, increasing the plant size and carbon inventory and thus the cost of the adsorption circuit
- Copper-loaded carbon can also result in significant copper reporting to dore bars along with gold and silver, increasing refining costs
- The copper-bound cyanide inventory in the heap can create environmental issues with heap closure that may require more extensive washing and possibly even cyanide destruction treatment, adding significant backend cost to the project.
Nearly all copper oxide minerals show significant solubility in cyanide (Hedley and Tabachnick, 1958; Marsden and House, 2006). Many sulphide copper minerals also show significant solubility, although less so than the copper oxide minerals. The overall cyanide-soluble copper fraction of a particular ore is best estimated through bottle roll and/or column test work, as the exact mineralogical composition cannot be conveniently determined.
In an operating cyanide heap leach, copper will begin to leach from fresh ore on the heap, and if unchecked can build up in solution to levels that begin to affect gold recovery and cyanide consumption, in turn affecting project economics. The problem of copper leaching is compounded when multiple lifts are placed on the heap (as is usually the case). Old lifts buried under active leaching areas can continue to slowly leach copper and consume free cyanide in the process. It is possible to install impermeable liners on top of old lifts, but this is expensive, technically difficult, and usually results in reduced overall gold recovery.
While each operation varies, a good general guideline is that if laboratory work indicates that field process solutions will stabilize at copper concentrations higher than 500 ppm Cu, gold recovery might be affected and copper control should be considered. Without some form of copper removal or treatment, the copper concentration in a typical heap leach process solution (in grams copper per litre) as a rule of thumb will build up to between three and six times the amount of leached copper (in grams copper per ton of ore) as determined in long-term laboratory column leach tests. This means that low-grade gold ores, especially those below 1 g/t Au, showing as little as 100 g/t leachable copper might be in need of copper treatment.
As an example, the additional cost of sodium cyanide attributed to leaching copper at a rate of 200 g Cu per ton of ore would be US$1.25 per ton of ore. This assumes a cost of US$2.50 per kilogram NaCN and an average consumption of 2.5 kg NaCN per kilogram Cu leached. To look at the cost another way, if this 200 g/t cyanide-leachable copper was present in an ore with a recovery of 0.5 g/t gold, the cash cost of the copper-bound cyanide consumption alone would be US$78 per ounce Au (it takes about 62 t of ore to produce one ounce of gold at this recovery).
This illustrated cost does not include the cost of cyanide destruction, which if no copper/cyanide recovery treatment is proposed, may be required for some projects at high copper concentrations. As a rule of thumb, the cost of cyanide destruction is roughly equal to the cost of purchased cyanide, so the above costs would effectively be doubled if cyanide destruction is required.
For proper heap design for cyanide leaching of copper-bearing gold ores, it is necessary to run long-term column tests (typically 60 to 180 days) at two or three different cyanide and/or pH levels. The relationships between gold- silver-copper recoveries, cyanide levels, and leach times will be different for each orebody. Since gold-copper types of orebodies tend to be emplaced in large acidic volcanic systems, ore characteristics can be variable and more than one sample may need to be tested. It is important that the test programme be comprehensive and defined early in the project evaluation process.
General methods of copper removal from cyanide solutions
Several methods have been proposed to treat copper in precious metal cyanide solutions, detailed descriptions of which can be found elsewhere (Briggs and Kidby, 1990; Fleming et al., 1995; Botz and Parodi, 1997, MacPhail and Fleming, 1998; Barter, 2001; Marsden, 2006; Botz and Acar, 2007; Ford et al. 2008, Guzman et al. 2010). Some of the more established or piloted methods include the acidification-volatilization-recovery (AVR) method, electrowinning, and sulphide precipitation (e.g. MNR and SART). Ion-exchange processes (e.g. AuGMENT and Vitrokele) can be used as pre-concentration steps in combination with these recovery methods. Table I provides a brief description of these processes.
Of the above processes, SART is most commonly encountered in operations, and will be discussed in the following sections.
SART process for copper removal and cyanide recovery
General process
The SART process recovers copper, and other metals such as silver and zinc, as a sulphide precipitate, separates the precipitate from solution, and recovers the cyanide that was bound to the copper by re-establishing an alkaline pH to the effluent (neutralization). The neutralized solution is recycled to the leaching process.
SART process chemistry is in essence simple and is summarized by the following reactions:
Sulphidation and acidification:

significantly complete at pH < 5.0
Neutralization:
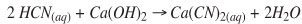
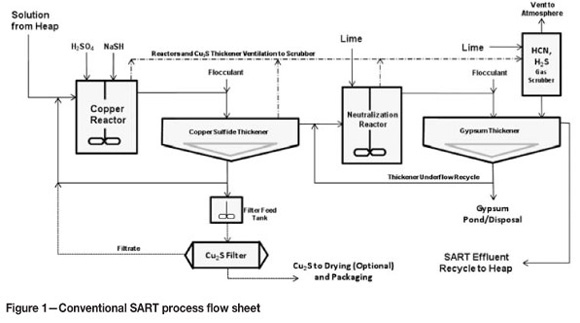
The conventional SART process flow sheet is presented in Figure 1. It is thought by the authors that this traditional approach has resulted in the construction of some SART plants that may have been more complex or expensive than is necessary. The 'SART LITE' flow sheet is presented as a possible alternative to the conventional SART circuit in Figure 2. The properly engineered flow sheet needs to fit each specific project, so it is recognized that some combination of the two flow sheets might be appropriate in some cases.
SART plant unit operations
This section will examine some aspects of SART plant design and suggest areas where the design might be simplified and the capital costs reduced. Where equipment design or flow rate examples are used for illustration, a basis of a 20 000 t/ day heap leach is used, containing 200 ppm copper cyanide and 100 ppm free cyanide, treated entirely through SART.
Copper reactor
Heap leach solutions are typically maintained at a pH of 9.5-11.0. Incoming solution is pumped to a tank to which sulphuric acid is added to maintain a pH of 4.0 to 4.5. NaSH is added to precipitate copper mostly as chalcocite (Cu2S). The stoichiometric requirement for NaSH is 0.44 kg NaSH per kilogram Cu in the effluent (or 0.09 kg NaSH per cubic metre in the example). Typically a slight excess of NaSH is applied to account for other NaSH consumers (e.g. silver, oxygen, zinc). Under controlled conditions typical for the SART process gold will not precipitate, but a large percentage of silver will co-precipitate as silver sulphide with the copper sulphide.
Laboratory tests were run to evaluate the 'copper reactor' activities and indicated the following:
- It does not seem to affect the reaction or the physical nature of the precipitate if the NaSH is added either before or after acidification of the influent alkaline solution
- With effective mixing, the reactions take place within a few seconds once the appropriate pH is achieved
- NaSH reagent is expensive and excess NaSH can react with cyanide to produce the thiocyanate anion (SCN-), so the best proposed practice is to use about 95% of the required NaSH and accept a small amount of copper recycle to process
- More dilute Cu feeds produce more finely divided precipitate with poorer settling properties.
In the SART LITE flow sheet presented in Figure 2, the copper reaction tank has been eliminated. The reactions can take place in the pipe between the process feed pump and the copper thickener, where the large volume of solution will serve to smooth out any concentration gradients.
Copper sulphide thickener
The copper sulphide formed is very fine and forms very quickly. Recycle of 'seed' crystals does not appear to result in larger crystal growth. However, recycling of thickener sludge along with flocculent and/or coagulant addition does result in the formation of flocs that settle at least ten times the rate of the unconditioned raw precipitate at dilute copper concentrations (e.g. <200 ppm Cu). The settling issue of unconditioned raw precipitate, and thus the need for recycle and proper flocculent addition, becomes increasingly significant with decreasing Cu concentration in the feed. In general, underflow recycle and flocculent addition are critical design features necessary for economical thickener design.
The operating conditions of the copper sulphide thickener are different from those of a typical mineral plant thickener where ore slurries are thickened. In the SART copper sulphide thickener, the slurry contains only 0.1% to 1.0% solids (including the recycled copper sludge). The incoming stream can be introduced above the settled bed, and separates quickly from the 'densified' solids. A small-diameter, tall thickener (high-density thickener) will likely be more economical than a traditional thickener, and a design based on de-entrainment to create a thin clear overflow will be more economical than a 'traditional' design based on solids settling velocity. This is important because this thickener must be constructed with corrosion-resistant materials, and must be covered to capture HCN gas. The deep cone of a high-rate thickener is also important for inventory control and densification of the small amount of copper sulphide produced.
Therefore in the SART LITE flow sheet the conventional thickener has been replaced with a small diameter, high-density thickener.
Copper sulphide neutralization
A relatively small volume of copper sulphide slurry discharges from the bottom of the thickener, about 6-7 m3 per day of a 40% solids slurry from a heap leach processing 20 000 t of ore per day, as in the example. The slurry should be conditioned (made alkaline to pH 10) with the addition of lime or caustic on its way to the precipitate filters. This is an important step since there is always free or combined cyanide in the precipitate, and filter operations usually result in discharge of air as the cake is dried. If the cake was left acidic, the air would contain potentially dangerous levels of HCN gas. The neutralization reaction is rapid and can be done in the pipe leading from the thickeners to the filters. NaOH is the preferred neutralization reagent, since lime would introduce a sulphate precipitate, thus lowering the value of the concentrate. There is no need for a separate 'filter feed tank', since the thickeners provide a large reservoir of thickened sulphide pulp.
Lime neutralization reactor
In the traditional SART circuit, a stirred tank is included in which lime is added to the acidic copper-free solution. This is a traditional mineral industry approach, but a tank may not be needed in all cases. Modern in-line pH controls and peristaltic metering pumps permit accurate addition and mixing to ensure the slurry is at a pH of 10.5 or higher. The reaction is rapid and can be done in a pipeline. Therefore in the SART LITE flow sheet this tank has been eliminated. In the example about 0.50 kg lime per cubic metre is required for neutralization.
Gypsum thickener
In traditional SART plants a gypsum thickener is included in the flow sheet to account for the fact that some gypsum may form during the neutralization process (as both calcium and sulphate ions are introduced by acid and lime additions). The gypsum thickener has been eliminated from the SART LITE flow sheet, and although it may be appropriate to include in some flow sheets, its inclusion should not be automatically assumed. The level of lime added following copper recovery is not enough to create a saturated solution of gypsum. While in most cases the solutions are already saturated, the gypsum supersaturates very easily and comes out of solution very slowly. A potential question is whether enough of the gypsum will remain in the thickener, or whether the thickener simply provides a random place where some of the gypsum collects. A more appropriate place to collect gypsum may be in the pregnant or barren ponds, where it has additional time to crystallize and settle. The ponds are normally quite large and the volume of gypsum created over several years of operation can be collected there without a significant increase in pond capacity.
Flow sheets that may permit elimination of the gypsum thickener include:
a) Intercept pregnant solution off the heap in a tank, process this solution through SART, and discharge to the pregnant pond. Gypsum will settle out along with the normal sludge that collects in the pond, and the clear solution can be processed through carbon columns (or Merrill Crowe) as usual for gold extraction
b) Process pregnant solution via SART on its way to the carbon columns, use anti-scalant in large enough dosages to prevent gypsum precipitation onto the carbon
c) Process barren solution after carbon columns via SART and use the barren pond as the settling reservoir for the gypsum and, the most interesting but (so far) least evaluated -
d) Process pregnant solution through SART, but leave it acidic as it goes through the carbon columns. Then raise the solution pH to the alkaline side and discharge it to the barren pond. Carbon gold loading is known to be significantly higher in acidic than in alkaline solutions. Additional research is needed to validate the overall practical loading/stripping process in such a condition.
Gas scrubbing and plant safety
Both SART and SART LITE contain a gas scrubber. It is a common misconception that HCN(g) will rapidly gas out of an acidified cyanide solution, but in fact hydrogen cyanide is highly soluble in water. Therefore, the tank vent system and the scrubber need to be designed only to ensure a slight negative pressure in the appropriate vessels. In the case of SART the scrubber is venting two reaction tanks and one thickener, whereas in SART LITE it is venting only the (smaller diameter) thickener. With proper design of the thickener, the volume of exhaust air needed for scrubbing HCN(g) is very small (probably 200 CFM, or 400 m3/h). A small ventilation fan and small packed scrubber (1800 mm diameter, 5000 mm high) will provide adequate security for the example SART LITE plant.
It is important to not trivialize the dangers posed by an acidified cyanide solution. Regardless of the amount of ventilation, enclosed freeboard spaces in all vessels should be considered very hazardous. The plant (or the operators) should be fitted with HCN(g) monitors with alarms, and procedures for loss-of-power events and maintenance events need to be rigorously designed and implemented.
Control of reagent feeds
The major areas of chemical control within the SART plant are:
pH reduction from alkaline conditions to acidic using sulphuric acid. The operating pH range is 4.0-4.5. Excursions above this range reduce copper precipitation, and excursions below this range can increase precipitation of gold and form undesirable copper precipitates such as CuCN and CuSCN
Copper precipitation with NaSH. Control of this reagent is very important. NaSH is expensive, and excess NaSH will consume free cyanide to form thiocyanate. The best control philosophy seems to be to use a slight deficiency of NaSH so the discharge of the SART plant still contains a minor amount of copper. Commercial instantaneous on-line analysers for copper (including cuprous ion) are limited. Fortunately, in most heap leach solutions the copper concentration fed to SART will be fairly consistent as it is averaged over a large volume of solution, so off-line analysis of Cu for NaSH control may be satisfactory in some of these cases
pH adjustment to alkaline upon solution discharge using lime. The only important consideration in this control is to achieve a pH above 10.5 to fix the regenerated cyanide
Flocculent and de-scalant usage. Flocculent will be used in the copper sulphide thickener, and de-scalant may be used at more than one point in the process. These can be evaluated in the laboratory prior to plant design, but the final use will be determined once the plant is in operation
Sodium hydroxide for neutralizing copper sulphide precipitate at a pH of about 10. This control is mainly for safety purposes. Residual cyanide in the copper sulphide sludge fed to the filter presses is very small and will cause minimal if any re-dissolution of copper
Sodium hydroxide for HCN gas scrubbing.
SART plant economics
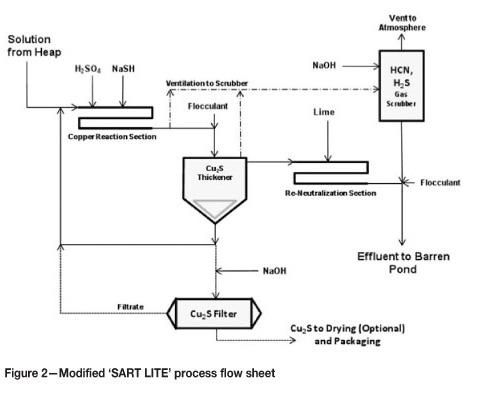
SART estimated operating costs
For illustrative purposes, a breakdown of SART plant operating costs is provided in Figure 3. In this example the design is based on an arbitrary 20 000 rrrVday influent (833 m3/h) containing 200 ppm Cu and 100 ppm free NaCN, which is the same basis for which the design considerations in the previous section were treated (i.e. a medium sized heap leach of 20 000 t/day containing 200 g Cu per ton ore leached, treated entirely through the SART circuit).
Reagent costs used are US$1.00 per kilogram NaSH, US$0.125 per kilogram acid, and US$0.15 per kilogram lime, assuming average transportation costs (i.e. reasonably good access to site). Power cost is assumed at US$0.10 per kilowatt-hour. Labour costs reflect a fairly high amount of plant automation (automatic control of reagents and flows) and thus minimal staffing.
As shown in Figure 3, total cost of the SART treatment in the example is US$0.45 per cubic metre. At 200 ppm (200 grams Cu per cubic metre) copper recovered, the plant breaks even at a net realization cost for copper in the precipitate of about US$3.60 per kilogram copper. This value includes a US$1 per kilogram transport-smelting-refining charge for the Cu, and gives no credit to the value of the recovered cyanide.
In terms of cyanide saved, at a price of US$2.50 per kilogram NaCN, the plant in the example needs only to recover about 50% of the influent copper-bound cyanide to cover operating costs. A properly designed SART plant should routinely recover 80-95% of copper-bound cyanide.
With high prices for copper (US$7 per kilogram assumed in this case) and costs for cyanide in recent times, a SART plant may actually be an ancillary profit centre for a project.
Table II illustrates the potential savings/benefits of SART in different terms of cash cost of Au produced, at different copper grades at a gold grade of 0.5 g/t on a recovered basis, using the same assumptions as above (for a 1 g/t Au grade the costs would simply be halved).
SART estimated capital costs
For illustrative purposes, a breakdown of a SART plant capital costs is provided in Figure 4. This breakdown is based on the same general assumptions as for estimating the operating costs. A 1000 m3/h plant is assumed. Costing is estimated from a general equipment list that reflects a standard SART plant design. The costs do not include any infrastructure requirements such as water, power supply, etc.
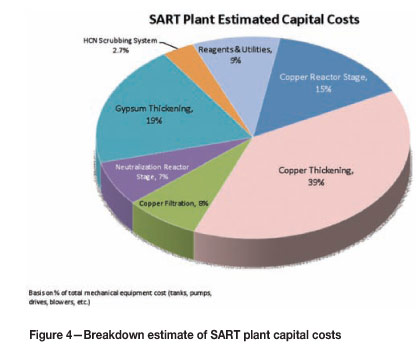
As can be seen from Figure 4, almost 40% of the total mechanical equipment cost is accounted for by the copper thickener alone (tank, rake, associated pumps and equipment), and about 60% of the total cost by the two thickeners together. This indicates that proper copper thickener design and careful consideration to gypsum disposition (e.g. determining if the gypsum thickener is even necessary) are keys to optimizing the plant cost.
When considering the traditional SART flow sheet, an installed turnkey SART plant at the 1000 m3/h size might be expected to cost somewhere between $750 and $1500 per daily cubic metre treated. A SART LITE plant would cost about 65% of a traditional SART plant. It might not be possible to incorporate all the cost savings of a SART LITE plant, of course, but keeping an open mind to a 'value-oriented' engineering approach to the design could in some cases result in a better overall project than to simply follow the conventional approach.
References
BARTER, J. 2001. Cyanide management by SART. Cyanide: Social, Industrial and Economic Aspects, TMS (The Minerals, Metals & Materials Society), Warrendale, PA. [ Links ]
BOTZ, M. and ACAR, S. 2007. Copper precipitation and cyanide recovery pilot testing for Newmont Yanacocha Project. 2007SME Annual Meeting, Salt Lake City, Utah, 25-28 February 2007. SME Preprint 07-036. [ Links ]
BOTZ, M.M. and PARODI, G. 1997. Removal of copper from cyanide leach solutions. Presented at the Randol Copper Hydromet Roundtable, Vancouver, British Columbia. pp. 217-219. [ Links ]
BRIGGS, A. and KIDBY, D. 1990. Cyanide recovery using Vitrokele technology: projected economic analysis. Randol Gold Forum '90, Squaw Valley, CA. pp. 325-328. [ Links ]
DAI, X. and BREUER, P. 2010. Modeling the equilibrium loading of gold onto activated carbon from complex cyanide solutions. Journal of Minerals and Metallurgical Processing, vol. 27, no. 4, November. [ Links ]
DAI, X. 2010.A mechanistic model of the equilibrium adsorption of copper cyanide species onto activated carbon. Hydrometallurgy, vol. 101. pp. 99-107. [ Links ]
FLEMING, C. and NICOL, M. 1984. The adsorption of gold cyanide onto activated carbon. III. Factors influencing the rate of loading and the equilibrium capacity. Journal of the Southern African Institute of Mining and Metallurgy, vol. 84, no. 4. pp. 85-93. [ Links ]
FLEMING, C.A., GROT, W.G., and THORPE, J.A. 1995. Hydrometallurgical extraction process. U S patent 5,411,575. [ Links ]
FORD, K., HENDERSON, R., and FLEMING, C. 2008. Application of the SART Process to heap leaching of gold-copper ores at Maricunga, Chile. 40th Annual Meeting of the Canadian Mineral Processors. [ Links ]
GUZMAN, G., MAMANI, V., AREVALO, H., VICUÑA, S., VARGAS, L., and BURGER, B. 2010.SART/AVR Circuit design and operation at Yanacocha Gold Mill. Precious Metals '10, Falmouth, UK, 15-16 June 2010. [ Links ]
HEDLEY, N. and TABACHNICK, H. 1958. Chemistry of cyanidation. Mineral Dressing Notes 23, American Cyanamid Company, New York. [ Links ]
MACPHAIL, P. and FLEMING, C. 1998. Cyanide recovery by the SART process for the Lobo-Marte Project, Chile. Randol Gold and Silver Forum, Denver, CO., 26-29 April 1998. [ Links ]
MARSDEN, J. and HOUSE, I. 2006. The Chemistry of Gold Extraction. Ellis Horwood, Chicheseter, UK. [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2012. ISSN2225-6253. This paper was first presented at the Percolation Leaching Conference, 8-9 November 2011, Misty Hills, Muldersdrift.














