Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 n.12 Johannesburg Jan. 2012
Talvivaara bioheapleaching process
P. SaariI; M. Riekkola-VanhanentII
ITalvivaara Sotkamo Mine Ltd
IITalvivaara Mining Company Plc
SYNOPSIS
The Talvivaara deposits are located in Eastern Finland, within the Kainuu Schist Belt. These polymetallic sulphide deposits are of relatively low grade, but bioheapleaching enables economically profitable nickel extraction from the ore. Zinc, cobalt, and copper are also produced. Talvivaara's production process begins with large-scale open-pit mining and four crushing stages, followed by stacking of the ore in bioleaching heaps. The ore is first leached for 13-14 months on the primary leach pad, after which the leached ore is reclaimed, conveyed, and re-stacked on the secondary heap pad. After secondary leaching, the barren ore will remain on the secondary heaps permanently. In the metals recovery process, the metals are precipitated from the pregnant leaching solution. Construction of the process was begun in spring of 2007 and first metal sulphides were produced at the plant in October 2008. The development of the bioheapleaching process was begun in a 17 000 t on-site pilot heap operated during 2005-2008 and has continued in the industrial heap. At industrial scale, the development measures have focused on improving the permeability and aeration of the heap. Challenges with crushing and aerations systems at the beginning of industrial-scale leaching delayed the increase in metal recovery, but after the first two operational years the leaching results have improved significantly and the process is performing as targeted.
Keywords: bioheapleaching, polymetallic sulphides, nickel, process development, aeration, permeability.
Introduction
The Talvivaara deposits are located in the southern part of the Kainuu belt in Eastern Finland (Figure 1). They comprise two different polymetallic ore bodies. The ore is relatively low grade, but bioheapleaching enables economically profitable nickel extraction from low-grade ore. At present, Talvivaara is estimated to be among the twelve largest nickel producers in the world, and once the planned production level of 50 000 t has been reached, the Sotkamo mine will be one of the world's six largest nickel mines.
Project history
The Geological Survey of Finland (GSF) carried out detailed exploration in the Talvivaara area from 1977 to 1983. As a result of this work, two polymetallic deposits, Kuusilampi and Kolmisoppi, were established. Outokumpu Plc was granted mining licenses to the Talvivaara deposits in 1986 and continued the geological work in the late 1980s and early 1990s. The resource was found to be large but of relatively low grade. The Talvivaara deposits remained unexploited until the Talvivaara Project acquired the rights to the deposits in February 2004 and continued the geological work by focusing on sampling for metallurgical purposes and investigating sample areas for the pilot trial.
With the deposits, Talvivaara was assigned the right to use all exploration data and research documentation relating to various process options studied. Bioheapleaching seemed to be the most economical option. Construction of a 17 000 t on-site pilot heap was started in May 2005 and initial bioheapleaching was started in August of that year. A metals recovery pilot campaign using the pregnant leaching solution (PLS) from the process was run in 2006 in the OMG plant in Kokkola. The environmental permit was granted in March 2007 followed by the commencement of the construction phase of the project. In April 2008 ore mining started at Kuusilampi open pit and in July 2008 bioheapleaching was initiated. The first metal sulphides were produced at the plant in October 2008.
Deposits
The Talvivaara deposits are located in Eastern Finland, approximately 350 km south of the Arctic Circle within the Proterozoic Kainuu Schist Belt. The Ni-Cu-Co-Zn mineralization at Kuusilampi and Kolmisoppi is hosted almost entirely by high-grade metamorphosed and intensively folded black schists. The main mineral assemblage in the black schists is quartz, biotite, graphite, and sulphides. Sulphide content in the ore is typically from 15 to 25 per cent and the valuable metal-containing sulphides are pyrrhotite, pyrite, pentlandite, sphalerite, violarite, and chalcopyrite. The average content of the ore using a cut-off of 0.07% of nickel is 0.23% Ni, 0.50% Zn, 0.13% Cu, and 0.02% Co. The distribution of nickel in different sulphides is pentlandite 66%, pyrrhotite 33%, and pyrite 1%. The distribution of cobalt is pentlandite 11%, pyrrhotite 26%, and pyrite 63%. All copper is in chalcopyrite and zinc in sphalerite. The Talvivaara deposits comprise one of the largest sulphide nickel resources in the world, with 1121 Mt in the measured and indicated resource categories (Table I). In addition to nickel, zinc, copper, and cobalt the ore contains about 0.3% manganese, 10% iron, 9% sulphur, 8% carbon, and 50% SiO2.
Production process
Overview
Talvivaara's production process (Figure 2) begins with large-scale open-pit mining, followed by materials handling which covers all the physical ore processing steps from primary crushing to stacking of the ore in bioleaching heaps. The ore is first leached for 13-14 months on the primary leach pad, after which the leached ore is reclaimed, conveyed, and re-stacked on the secondary heap pad. After secondary leaching, the barren ore will remain permanently on the secondary heaps. In the metals recovery process, the metals are precipitated from the pregnant leaching solution.
Open-pit mining
Ore and waste rock are extracted using conventional open-pit drill and blast methods. The planned annual ore production is approximately 24 Mt. The average waste to ore ratio over the life of mine is estimated to be very close to 1. Any overburden or moraine not required for road, perimeter wall, or other construction is stockpiled for later use in rehabilitation.
Materials handling and bioheapleaching
After primary crushing, the ore is conveyed to a fine crushing station, where it is crushed in three further stages so that 80 per cent of the resulting particles are approximately 8 mm or less in size. The particle size of the ore plays an important role in the process, with small particle sizes correlating with fast leaching. In industrial heap leaching operations, however, very small particle sizes are often not desirable, largely due to high crushing costs and problems in heap stability and permeability with finely crushed ore. Before pilot heap construction, column experiments were performed with the Talvivaara ore with particle sizes 6, 8, and 12 mm. The 8 mm particle size was chosen for the pilot and industrial leaching because nickel recovery with the 12 mm particle size was 45 per cent less when compared to results of the column with the 8 mm particle size. The crushed ore is then agglomerated in a slowly rotating drum, where PLS is added to the ore in order to consolidate the fine ore particles with the coarser ones. This preconditioning phase makes the heap permeable to air and water and promotes the start of the bioleaching reactions.
After agglomeration, the ore is conveyed and stacked as 8 m high heaps on the primary heap pad for 13-14 months of primary leaching, after which the leached ore is reclaimed, conveyed, and re-stacked onto the secondary heap pad, where it is leached further.
The heap pad (Figure 3) is equipped with piping through which low-pressure fans supply air to the stacked ore. From the top, the heap is irrigated with leaching solution, which is collected from the bottom of the heap with a drainage system and discharged to the PLS collection ponds. Once a sufficient metal concentration is reached, a continuous side stream (about 10-20 per cent) of PLS is pumped to the metals recovery plant and the rest of the solution is circulated back to the heap. Either fresh water or purified process water is added to the circulation to maintain process water balance and appropriate leaching conditions. The pH value of the irrigation solution is adjusted with sulphuric acid.

Metals recovery
In the metals recovery process, the metals are precipitated from the PLS using gaseous hydrogen sulphide. The resulting products are metal intermediates; copper and zinc sulphides, and a mixed nickel-cobalt sulphide. These intermediates are transported for further processing in refineries operated by Talvivaara's customers.
Bioheapleaching at Talvivaara Pilot plant operation
The suitability of bioheapleaching technology for the extraction of metals from the Talvivaara deposits was verified in a 17 000 t on-site pilot heap operated during August 2005 - November 2008. The primary leaching of the 8 m high pilot heap (heap pad of 50 m x 100 m) took one and a half years and yielded nickel recovery well in excess of 90 per cent. In February 2007 the primary heap was reclaimed and restacked to the secondary heap and the bioheapleaching process was started again.
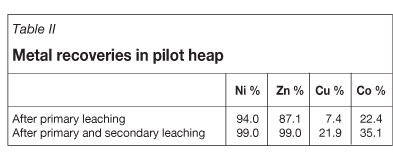
Secondary leaching was continued until November 2008, when the pilot heap had to be reclaimed as the Kuusilampi open pit was expanding to the site of the heap. The final nickel and zinc recoveries after 21 months of secondary leaching are presented in Table II.
The main reason for installing a secondary leaching phase is to obtain better recoveries of copper and cobalt. Copper is in chalcopyrite and the major part of the cobalt in pyrite. As sulphide minerals have semiconductor properties, galvanic interactions appear when there is an electrical contact between mineralogical phases. During dissolution of a mineral assembly of different sulphides, those minerals that have the highest rest potentials behave as cathodes, which means that they are galvanically protected and their leaching is hindered until the minerals with lower rest potentials have been leached. The electrochemical potentials of chalcopyrite and pyrite are higher than those of pyrrhotite, pentlandite, and sphalerite.
Removing the primary leaching material to the secondary leaching stage also enhances the recovery of metals from those parts of the primary heaps where leaching solution has had poor contact with the ore particles. Such areas include, for example, the slopes of the heaps. After secondary leaching, the barren ore is expected to remain permanently on the secondary heap area.
Industrial plant description
The primary heap of the industrial plant comprises two heap pads, a lower part and an upper part, with a 40 m wide corridor between them reserved for a service area, pipelines, and two fixed conveyor lines (Figure 4). The heap pads are 2400 m long and 400 m wide, and the entire surface area is 210 hectares. The heap pads are further divided in four sections. The heap pads are designed so they slope toward the PLS ponds. The cross slopes vary from 2 per cent to 3.5 per cent.
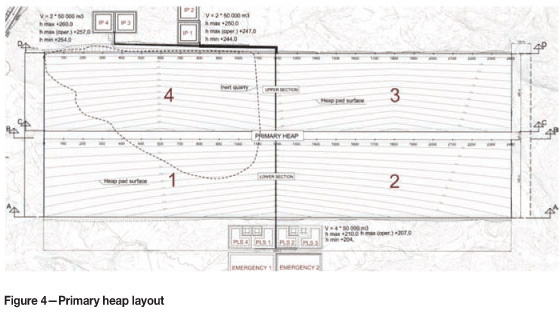
The primary heap pad at Talvivaara is constructed as a dynamic, or on-off, pad. This means that after the first stacking cycle, the ore is reclaimed using excavators and conveyed to the secondary leach pad for further leaching. While the excavators move ahead reclaiming the ore, the stacker follows behind them, stacking new ore from the open pit. This cycle is continuous.
The secondary leaching pads are constructed on top of waste rock dumps. The benefit of this arrangement is multiple lining systems and reduced earthworks. It also reduces the final footprint of the operation and final rehabilitation costs.
Both the heap foundations and the waste dumps are lined with bentonite and plastic layers to prevent leakages.
Crushed and agglomerated ore is stacked to the leaching pad and leaching is activated by the irrigation and aeration of the ore heaps. Several physico-chemical and microbiological process parameters, such as the pH of the irrigation solution, the rate of irrigation, the rate of removal of PLS, and the rate of aeration are continuously monitored and modified in order to enhance and speed up the metals recovery process.
The primary heap is irrigated using drip irrigation at a rate of 5 L/m2/h. The pH value of the irrigation solution is adjusted to 1.7-2.0 and the solution is circulated evenly on the heap surface. The solution percolates through the heap, allowing metals to dissolve into the solution. The pH of the irrigation solution is kept above 1.5 to prevent the dissolution of silicates. Dissolved silicates form a gelatinous layer which makes the heap impermeable. The PLS from the heaps is analysed for pH, redox potential, total iron concentration, ferrous iron levels, and metal concentrations.
The bioleaching bacteria require oxygen, which is the electron acceptor in the leaching process. Carbon dioxide is also important, because the bacteria need it as carbon source for their growth. Carbon dioxide is usually not a limiting factor in bioleaching, because it is a constituent of the air used for aeration and it is produced by dissolution of the carbonate minerals from the ore. However, oxygen can become a limiting factor. The amount of air needed is calculated based on the amount of ore to be leached, the amount of sulphide to be oxidized, and the bioleaching time. An efficiency factor is included in the calculation formula. The aeration system for the primary heap consists of 32 low-pressure fans which have been installed in movable containers.
Bioleaching micro-organisms
In nature, bioheapleaching is triggered spontaneously by naturally-occurring micro-organisms in the presence of air and water. The outcome is usually seen as the slow weathering of sulphide-containing orebodies. Commercially applied bioheapleaching technologies accelerate this natural process. The bacteria used in the Talvivaara process grow naturally in the ore and the process of bioleaching is made technically viable by creating optimal conditions for growth of the bacteria. Inoculation of the heap is not performed.
The micro-organisms active in bioleaching heaps are iron- and sulphur-oxidizing bacteria and archaea. These microbes share several common features making them suitable for bioleaching. The most important of these characteristics include:
Autotrophic growth by fixing CO2 from the atmosphere
Energy obtained by using either ferrous iron or reduced inorganic sulphur compounds or both as the electron donor, and generally using oxygen as the electron acceptor
Acidophiles that grow in low pH conditions, typically optimum pH 1.4 to 1.6
Remarkable tolerance to a wide range of metal ions, although there is considerable variation within and between species.
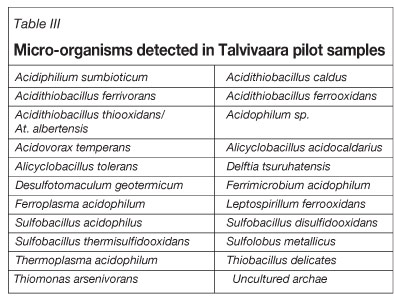
Bioleaching micro-organisms include mesophiles (organisms that grow at temperatures up to 45°C), moderate thermophiles (organisms that grow at temperatures up to 60°C), and thermophiles (organisms with their optimal temperature for growth above 60°C). The micro-organisms detected in Talvivaara samples are listed in Table III. The micro-organisms have been detected from liquid samples using DNA extraction and denaturing gradient gel electrophoresis (DGGE) analysis.
Temperatures in bioheapleaching
Typically, primary and secondary sulphide ores, such as those found at Talvivaara, are associated with pyrrhotite, which has the potential to release large quantities of heat when oxidized. This heat generation makes the bioheapleaching process well suited for the sub-arctic climate of Eastern Finland (Figure 5).

The large-scale pilot studies carried out at the mining site have demonstrated that the temperatures inside the heap remain roughly in the range of 20-90°C (Figure 6) regardless of the outside temperature, which in Finland can vary between -30°C and +30°C.
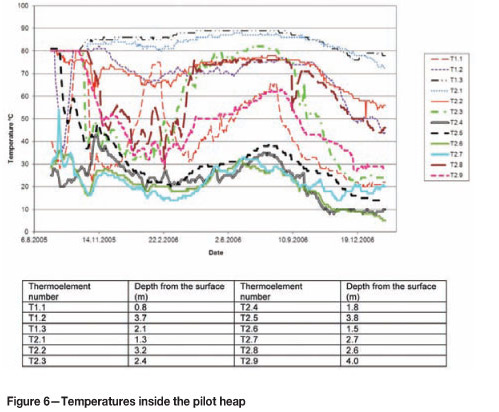
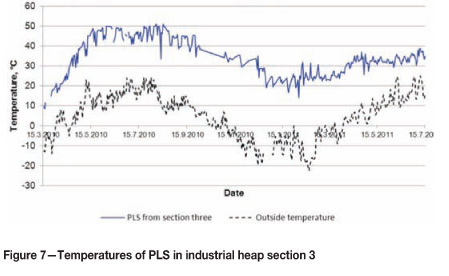
The same phenomenon is now also occurring in the industrial heaps. The temperature of the PLS exiting the heaps typically varies between 20-50°C. Figure 7 presents the temperatures of the PLS flowing out from primary heap section 3. The temperatures presented are typical of all primary heap sections with good leaching results.
Industrial-scale process development
Stacking of the first primary heap section was begun in July 2008. The stacking of the first section proceeded much too slowly due to difficulties in the crushing circuits. Irrigation and aeration could be started only when about 25 m of heap had been built, which in the beginning took 4-5 weeks, and the agglomerates started to break apart during that time. The particle size was also smaller than planned. When irrigation and aeration were started, there were difficulties in getting an even airflow through the heap. In 2009 the crushing process was modified by adding new crushing steps and coarser screens. The redesigned and enhanced crushing circuit was taken into operation at the beginning of September 2009. The modifications resulted in improved crushing capacity and more accurate control over the particle size distribution of the crushed ore. The improved crushing capacity and targeted particle size led to improved leaching results, with primary heap section 2 built between June 2009 and January 2010.
Process development was also targeted by altering heap height and the structure of the aeration system during the stacking of primary heap sections 1 and 2. Stacking was begun with heap height of 10 m, which was later changed to 8 m. In section 2, experiments were made also with lower heap heights (6 m and 4.5 m, with only bottom aeration). Aeration of the first section was performed by using one set of aeration tubing at the bottom of the heap. In section 2, the possibilities for improved aeration were tested by also installing intermediate aeration tubing in the middle of the heap (see Figure 9).

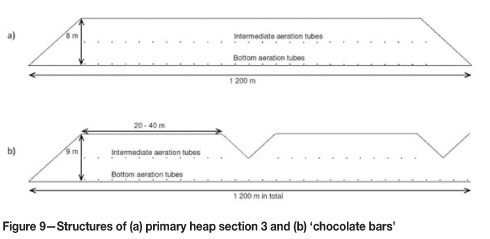
The leaching results obtained from areas with different structures in the first two heap sections were carefully monitored and the structure of the third section designed based on these findings. Efficient leaching was found to begin faster and the leaching rates were better in the areas with the heap height of 8 m and double aeration, compared to the areas of 10 m or 8 m height with only bottom aeration. Leaching of the sections with the height of 6 or 4.5 m had just began when making the decisions concerning the design of section 3, and no conclusions could then be drawn about the leaching efficiency of these lower heaps. Later on it could be concluded that the leaching rate was 10-25 per cent higher in the lower heaps, but considering also the fact that 25-44 per cent less material per square metre of leaching pad can be stacked when using lower heap heights, 8 m heaps are regarded as more optimal. Section 3 was thus stacked 8 m high and equipped with two layers of aeration tubing.
Based on the finding that the ends of the sections leach more efficiently than the rest of the material, another aeration experiment was designed for primary heap section 4. Part of this section was stacked as 'chocolate bar' structures (see Figures 8 and 9).
'Chocolate bar' heap sections are separated by ditches, i.e., lower heap sections. Different structure widths have been tested. The leaching of the material stacked as 'chocolate bars' is still ongoing, but the conclusion has been that the leaching reactions start more rapidly in these structures, probably due to enhanced aeration.
Current situation and conclusions
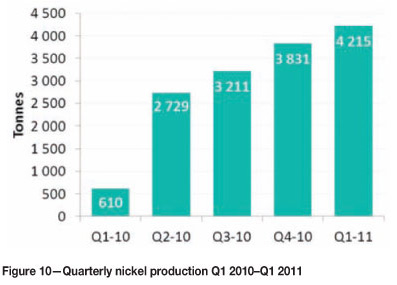
In process development, particular attention has been paid to improving permeability and aeration of the heap. With improved crushing and aeration systems, the bioheapleaching has started to progress according to expectations. The primary heap was fully stacked for the first time in November 2010, and secondary leaching started with good results. Nickel production at the metals recovery plant has increased steadily (Figure 10).
Metal concentrations in the PLS of the primary sections were under target until spring 2011 due to the challenges described above connected to the leaching of primary heap sections 1 and 2. These challenges led to the situation where the amount of PLS pumped from section 1 to the metals recovery plant was larger than in the original process design. The feed to the metals recovery process has been taken from the section with the highest nickel grade and, as seen in Figure 11, this delayed the increase in nickel concentrations in the PLS as the amount of nickel recovered (pumped out) from the section has been greater than the amount of nickel dissolved.
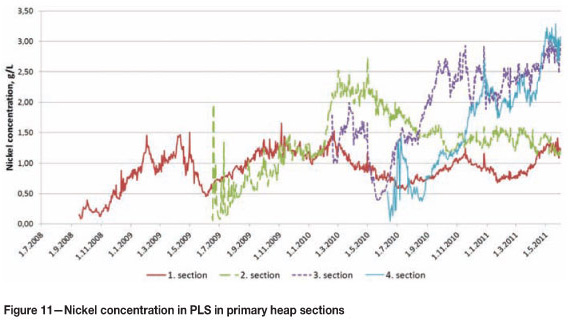
As a result of the process development measures, leaching results have improved significantly with primary heap sections 3 and 4, and nickel grades in leach solution have increased especially in these newer heap sections, reaching levels above 3 g/L. Start-up of the bioheapleaching process and the first operational years at industrial scale have provided a lot of valuable hands-on knowledge about the requirements of successful bioheapleaching of the Talvivaara ore. After this learning curve, the process can now be run according to the design parameters with targeted results.
Reference
RIEKKOLA-VANHANEN, M. 2011. Talvivaara Sotkamo Mine - Bioheapleaching of a polymetallic nickel ore in subarctic conditions. Proceedings of Nickel/Cobalt/Copper sessions, ALTA Conference, Perth, Western Australia, 23-25 May 2011. ALTA Metallurgical Services, Castlemaine, Victoria, Australia. pp. 150-163. [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2012. ISSN2225-6253. This paper was first presented at the Percolation Leaching Conference, 8-9 November 2011, Misty Hills, Muldersdrift.














