Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 n.12 Johannesburg Jan. 2012
Review of the role of microbiology in the design and operation of heap bioleaching processes
M. Gericke
Biotechnology Division, Mintek
SYNOPSIS
Over the past few decades the commercial application of heap bioleaching technology for the extraction of base metals has become increasingly important, due mainly to the depletion of high-grade ore reserves. Heap bioleaching is widely used for the extraction of copper from secondary copper sulphide ores. The design and engineering aspects of the process have received considerable attention, but issues related to the microbiology of the process have been subjected to less rigorous scrutiny.
The major role of micro-organisms in bioleaching processes is to catalyse the regeneration of ferric iron and protons, from ferrous iron and by sulphur oxidation respectively. It is accepted that even the most carefully engineered heaps are heterogeneous in terms of temperature, pH, the presence of anaerobic pockets, irrigation efficiency, and dissolved solutes. Since interactions between solution chemistry, mineralogy, and microbial populations exist in heaps, a better understanding of the correlation between microbial numbers and types with changes in these chemical and physical profiles with time would be beneficial during process design and operation of heaps, and could result in faster start-up times and higher metal recoveries.
This paper reviews the role of microbiology in heap bioleaching processes. Aspects such as microbial diversity, identification and monitoring of cultures, inoculation strategies, colonization behaviour, and tolerance to metals and salts are discussed, and the potential contribution of the knowledge to the improvement of the operation and design of heap bioleach processes assessed. Conclusions are drawn with respect to the role of genetic engineering, heap inoculation practises, and remaining areas for future heap bioleaching research and development.
Keywords: heap bioleaching, microbiology, process design, operation.
Introduction
Globally, high-grade metal reserves are becoming depleted and new technologies for processing progressively more marginal ores are required. The challenges faced by the metals industries include lower-grade deposits, increased ore complexity, less accessible deposits, and (in the base metals sector) the growing importance of the abundant low-grade resources of refractory chalcopyrite, which are resistant to leaching.
Traditionally, heap leach technology has been applied in the mining industry for the recovery of copper from copper oxide minerals, the cyanide leaching of silver and gold, and dump leaching of run-of-mine wastes containing copper. More recently, heap bioleaching has emerged as a competitive technology for the leaching of low-grade secondary copper sulphides such as chalcocite and covellite and for pyrite in refractory gold ores, where the leach process is aided by iron-and sulphur oxidizing micro-organisms (Brierley, 2008).
In recent years the research focus shifted to the development of heap bioleach processes for the treatment of primary copper sulphides, i.e. chalcopyrite. It is estimated that chalcopyrite accounts for approximately 70 per cent of the world's copper reserves (Córdoba et al., 2008), and whereas chalcocite can be bioleached quite effectively at temperatures only slightly above the average ambient temperature, temperatures above 60°C are needed to achieve acceptable copper extraction from chalcopyrite ores (van Staden, 2008).
The design and engineering aspects of heap bioleach processes have traditionally received considerable attention, but issues related to the microbiological aspects of the process have been subjected to less rigorous scrutiny. It is widely acknowledged that to advance the technology it is important to gain an improved understanding of the biological aspects of the process. In addition, with the increased focus on the treatment of chalcopyrite ores, where the maintenance of high operating temperatures is necessary, it is accepted that a smooth temperature progression across the operating temperature bands of the various types of microbes is essential, necessitating improvement of our knowledge of the microbes present in the heap and the effect of varied operating conditions on their growth rates.
In this paper, the role of microbiology in heap bioleaching processes will be reviewed. Aspects such as microbial diversity, identification and monitoring of cultures, inoculation strategies, colonization behaviour, and tolerance to metals and salts will be discussed and the potential contribution of the knowledge to the improvement of the operation and design of heap bioleach processes assessed.
Identification and monitoring of microbes involved in heap bioleaching processes
Characteristics of microbes found in bioleaching processes
A number of review articles have been published that have described the physiologies and phylogenies of acidophilic micro-organisms in detail (Rawlings, 2005; Rawlings and Johnson, 2007; Watling 2006). The major role of microorganisms in bioleaching processes is to catalyse the regeneration of ferric iron and protons, from ferrous iron and by sulphur oxidation respectively (Rawlings and Johnson, 2007). Irrespective of whether tank or heap processes are used, the micro-organisms that catalyse biomining processes are required to grow in an essentially inorganic, aerobic, low pH environment. The most important micro-organisms are autotrophic and utilize CO2 as a carbon source. Although the exact nature of the energy sources may vary from mineral to mineral, the micro-organisms grow by oxidizing reduced forms of sulphur or ferrous iron, or both. The pH within tanks and heaps may also vary, but is highly acidic and typically within the range 1.0 to 2.0.
The rather extreme conditions in stirred tanks and heaps mean that the number of micro-organisms that are likely to play a dominant role in biomining processes is limited (Rawlings, 2005). It is important to note that in all pilot- and full-scale biomining operations that have been examined, microbial consortia (mixed cultures) rather than single cultures have been found (Johnson, 2008). Acidophilic bioleaching microbes present in bioleaching processes generally comprise several species with widely different temperature optima and ranges and may be conveniently grouped into three groups; namely mesophiles (20-40°C), moderate thermophiles (40-60°C), and thermophiles (60-80°C) (Rawlings and Johnson, 2007).
In general, the types of micro-organisms found in heap leaching processes are similar to those found in stirred tank processes. The most important micro-organisms in bioleach processes that operate from ambient temperatures to about 40°C are considered to be a consortium of iron- and sulphur-oxidizing Acidithiobacillus ferrooxidans, the sulphur-oxidizing Acidithiobacillus thiooxidans and Acidithiobacillus caldus, and the iron-oxidizing Leptospirillumferrooxidans and Leptospirillumferriphilum (Rawlings, 2005; Rawlings and Johnson, 2007). At moderately thermophilic conditions At. caldus, L.ferriphilum, Sufobacillus-like bacteria, and
Ferroplasma-like archaea seem to dominate (Okibe et al., 2003), while species of Acidimicrobium may also occur in systems operating at temperatures around 50°C (Rawlings, 2005). Thermophilic consortia are typically dominated by archaea with species of Sulfolobus, Acidianus, and Metallosphaera being most prominent (Rawlings, 2005).
Monitoring and identification of bioleaching microbes
Why do we need to monitor, quantify, and identify the microbes in heaps? It is accepted that differences in parameters such as temperature, pH, and aeration in different parts of the heap and at different times of the heap lifetime could have an effect on the populations present (Johnson, 2008). Gaining a better understanding of the correlation between microbial types and numbers with changes in the chemical and physical profiles with time in the heap would be beneficial and could potentially assist in issues such as whether re-inoculation is necessary, which microbial cultures to add, whether the water quality is acceptable, and if the build-up of elements in the raffinate recycled to the heap could have an inhibitory effect on microbial performance. The increased understanding of the adaptation of leaching bacteria to changing conditions could potentially be a step towards achieving faster start-up and increased metal extractions.
While careful considerations are made in the design and engineering of heap bioleach operations, the microbiological aspects have been subjected to far less scrutiny and control (Rawlings and Johnson, 2007), and somewhat surprisingly, there have been relatively few accounts of the compositions and dynamics of microbial populations in biomining operations.
One reason for the dearth of information has been the lack of accurate and appropriate methods for analysing populations that are active in bioleaching environments. Until a few years ago, direct microbial counts and indirect measurements such as oxygen uptake rates, redox potential, pH, ferrous iron concentration, and temperature were used as an indication of the bulk activity of micro-organisms in the heap. In addition, microbial enrichments from solutions and ores have provided an initial view of micro-organisms associated with the process. It was, however, not known whether these cultured strains were the key players in the process (Brierley, 2001; Demergasso et al., 2005).
The development of new culture-independent molecular techniques, such as polymerase chain reaction (PCR), realtime quantitative PCR, denaturing gradient gel electrophoresis (DGGE), and fluorescent in situ hybridization (FISH), to detect and quantify populations, is a significant advancement, and these have become powerful tools to describe biodiversity and to follow changes in microbial consortia present in bioleaching systems without having to culture the micro-organisms (Demergasso et al., 2005; Coram-Uliana et al., 2006; Watling, 2006; Johnson and Hallberg, 2007; Remonzellez et al., 2007). These techniques do not, however, give an account of the viability of the organisms, and recently a method for the rapid assessment of active biomass in leach liquors based on the measurement of ATP concentration in test solutions was described (Okibe and Johnson, 2011), which could be a simple way of quantifying microbial activity.
A few examples of microbes identified in pilot- and commercial-scale heaps are presented in Table I. The results, in all cases, show the presence of a relatively small community of organisms, which is consistent with observations previously described (Rawlings, 2005; Rawlings and Johnson, 2007).
Studies on the microbiology of heap leach systems focus mainly on analyses of the liquid phases, i.e. pregnant leach solutions and raffinate (Johnson, 2008). This is due mainly to practical problems of representative sampling of large areas and various depths of heaps, and of obtaining unbiased extraction of microbes or their DNA from the minerals and iron salt precipitates. Since the micro-organisms are not only present in the liquid fraction, but are also attached to the ore particle surfaces, it is important to include the attached population when assessing the microbial composition within the heap. To enable collection of representative data, sampling techniques will require further optimization through research and development.
Whether to inoculate and with which organisms?
Microbial diversity is needed within a heap at different stages in its life cycle, and the challenge is to ensure that there is sufficient biodiversity within a heap to achieve optimal performance (Rawlings and Johnson, 2007). Heap bioleaching operations typically rely on natural colonization by indigenous microbial strains. However, with the continued drive to achieve maximum metal recoveries in ever shorter times, the need arises to promote microbial colonization throughout the heap in the shortest time period possible, and also to maintain a population appropriate to the conditions established in the heap over time (Watling, 2006).
The question therefore arises whether one should only rely on the observation that iron- and sulphur-oxidizing micro-organisms are naturally ubiquitous, or should one inoculate a heap with organisms that are not likely to be present originally. In general, mesophilic and moderately thermophilic acidophiles that grow from ambient to approximately 50°C appear to be widely distributed in naturally acidic environments (Rawlings and Johnson, 2007). However, in the case of chalcopyrite heaps, which need to be operated at increased temperatures to achieve the necessary copper extraction rate, a consortium of micro-organisms that grow well over a range of temperatures from ambient to thermophilic is required. Thermophilic microbes (60-80°C) are less likely to be as ubiquitous (Rawlings and Johnson, 2007) and it is generally accepted that thermophiles might not be able to survive until temperatures in the heaps reach thermophilic levels. An additional inoculation step would therefore be required. Examples of inoculation strategies described in literature are presented in Table II.
An additional aspect to bear in mind is that the management of heaps (especially in the case of chalcopyrite, when using temperatures between 50°C and 60°C) could be challenging due to the reduced biodiversity of acidophiles and decreased activity of the microbes in this temperature range (du Plessis et al., 2007). This temperature gap must therefore be carefully managed to ensure that the heap temperature reaches the thermophilic zone at which chalcopyrite is amenable to effective bioleaching. Since the presence of temperature-sequential microbial populations that can facilitate the heating of the heap from mesophilic through to thermophile temperatures is critical (Brierley, 2001), inoculation with thermophiles several months before optimal conditions for their growth have developed in the heap could affect the survival of these organisms negatively, and a second round of inoculation with thermophiles would probably be required (Dew et al., 2009).
The overall conclusion is that there would likely be a considerable time saving in inoculating a new heap with a microbial consortium (or consortia) at the appropriate points in time, rather than waiting for micro-organisms to grow naturally.
Which micro-organisms to include in an inoculum is another challenge. Questions which have been debated over the years include 'Is there a superbug that will accelerate bioleaching processes?' and 'Is there an ideal combination of micro-organisms?'
Two contrasting approaches have been suggested and investigated to determine whether it would be possible to produce an ideal or optimized consortium of bioleaching micro-organisms for a given process or substrate (Rawlings and Johnson, 2007; Johnson 2008). In the 'bottom-up' approach, the reference point is the rate of mineral oxidation/metal solubilization by a pure culture of one or more chemolithotrophic iron-oxidizing acidophiles such as a Leptospirillum sp. Mixed cultures containing additional acidophiles with complementary metabolic abilities, such as the ability to oxidize sulphur or to grow heterotrophically, are then compared. The aim is to identify a microbial consortium that is not only highly efficient at catalysing the oxidative dissolution of target minerals, but which is also stable and robust. The 'top-down' approach utilizes an inoculum that contains a wide variety of different species and strains of acidophiles, on the basis that the species that are most fit for bioleaching a particular mineral concentrate will survive, while those that cannot compete are eliminated. Bacteria and archaea that vary in e.g. energy and carbon sources, pH and temperature optima, and metal and solute tolerance are recommended for inclusion in the initial inoculum.
Although interesting results were obtained, it seems highly unlikely that it would be possible to maintain such a consortium in a full-scale heap leach operation given the fact that these processes are open, non-sterile systems. The best one can probably do is to ensure that sufficient biodiversity is present in the solutions used to inoculate the heaps.
Adaptation and genetic manipulation
Adaptation of bacteria to different conditions, such as increased tolerance to high metal levels, is a simple means of genetic improvement. The technique is dependent upon the small number of errors in the DNA sequence that are made during chromosomal replication. Most errors are harmful or neutral, but some may be advantageous. Thus, when a selective pressure is applied to a population, those bacteria that acquire an advantageous mutation will outperform the rest and dominate the population. For example, by growing bacteria in a continuous flow reactor under conditions of increasing flow rate, fast-growing bacteria will be enriched while slow-growing bacteria will be washed out. The advantage of 'mutation and selection' is that it can be applied in the laboratory without requiring specialized knowledge of bacterial physiology and biochemistry. The disadvantage is that it is a slow process and could take years to progress from environmental isolates to adaptation to rapid growth in highperformance biooxidation tanks (Watling, 2006; Rawlings, 2011).
Work began in the early 1980s on the development of genetic systems for biomining micro-organisms. Initially it was hoped that genetic engineering of biomining organisms would increase metal tolerance by insertion of genes resistant to metals that the organisms are currently sensitive to and reduction of certain metabolic bottlenecks by adding, for example, a more effective CO2-fixing enzyme (Rawlings, 2011).
Currently, however, the genetic engineering of mineral processing bacteria tends not to have a high priority among bioleaching researchers (Watling, 2006; Rawlings, 2011). Reasons for this include:
There is some doubt that engineered strains would be sufficiently robust to survive and compete effectively in the complex, non-sterile, open environments of bioleaching processes
There is a great deal of uncertainty about regulatory issues concerning the release of genetically engineered strains into the environment
Bioleaching processes operate best with a consortium of micro-organisms present, and if all members of the interdependent consortium have not been modified there may be no gain
In the case of heap leaching operations, these tend to be relatively low rate processes with an even greater diversity of microenvironments and micro-organisms. Under such circumstances the advantage of genetic modifications is likely to be reduced.
Genetic engineering may therefore have a much smaller role to play in biomining processes than originally envisaged. The main value of the development of genetic systems may lie in the ability to study the role and functions of different genes and their metabolisms, and will probably be important in the indirect improvement of bioleaching through an enhanced understanding of the genetics and physiology of the organisms, rather than directly by the addition of genetically manipulated micro-organisms to commercial processes. (Rawlings, 2011)
Microbial tolerance to high metal and salt concentrations
An important characteristic of the acidophilic chemolithotrophs is their general tolerance of high concentrations of metallic and other ions. The inhibition and the resistance mechanisms exhibited by several acidophilic bacteria and archaea to metal ions such as As3+, Cu2+, Zn2+, Cd2+, and Ni2+ have been reviewed in detail elsewhere (Dopson et al., 2003; Rawlings, 2005) and will not be discussed here. The fact that these organisms survive and thrive in bioleaching environments shows a remarkable ability to adapt to and tolerate the relatively high element concentrations they encounter. Nevertheless, strong bacterial tolerance does not necessarily result in increased metal extraction, and there are limits above which bacteria cannot adapt for reasons of toxicity and/or high ionic strength (Watling et al., 2009).
In heap leach processes, the relatively large quantity of gangue compared to valuable metals, continuous recycle of the solution inventory, and the prolonged times of exposure in the absence of deliberate removal strategies can result in the release of considerable concentrations of gangue cations in the heap leach solution, to the point where they exceed limits commonly considered toxic to bioleaching microorganisms. Elemental concentrations in heap leachates vary depending on the composition of the ore and process water, but are generally up to around 20 g/L for Fe, Al, and Zn, up to 10 g/L for Cu, Ni, and Mg, and up to 1 g/L for Co, Ca, Si, Na, and K. In addition, the presence of SO42- at concentrations higher than 120 g/L is not uncommon (Watling et al., 2009; Ojumu et al., 2006).
These values indicate that in every respect the solution conditions are far from what would be considered optimal in a typical bioleach operation. These salts create potentially adverse conditions for the microbial population and interfere with the microbial oxidation of ferrous iron, which is a critical sub-process in bioleaching. This could explain why in many heap bioleach operations the rate and extent of metal recovery remains below what could be achieved in theory, and it is postulated that this is due to the adverse solution conditions affecting microbial growth and activity (Ojumu et al., 2008).
The importance of comprehensive laboratory evaluation during development of an ore body to commercial processing using biohydrometallurgy can therefore not be understated. Laboratory evaluation must include rigorous evaluation of the microbiological component and definition of operating parameters that engineers should bear in mind when designing the commercial plant. Failure to meet commercial production at a mine site can be the consequence of the incomplete understanding of the biological component of the process. One such example has been described by Brierley and Kuhn (2010), where the inability of a copper bioleach process to meet the design criteria was in part due to a lack of sufficient testing to demonstrate the toxic effect of fluoride on the microbial component of the bioleach process.
A number of papers have recently been published describing the effect of fluoride toxicity on the performance of bioleaching micro-organisms (Sundkvist et al., 2005; Dopson et al., 2008; Gunneriusson et al., 2009; Brierley and Kuhn, 2010). It has been observed that fluoride originating from gangue minerals effectively inhibits bioleaching at concentrations as low as 0.1-1 mM (Sundkvist et al., 2005). The toxicity effect increases at lower pH levels due to the formation of hydrogen fluoride, which can easily penetrate the bacterial cell membranes. Strategies to reduce the concentration of free fluoride ions include addition of cations such as Al3+, which results in the formation of non-toxic complexes.
Aluminium, magnesium, and nitrate ions are often significant contributors to reduced water quality in bioleaching operations, and the potential inhibitory effect of Al and Mg on ferrous oxidation has been illustrated by amongst others Blight and Ralph (2008) and Ojumo et al. (2008). Ojumo et al., (2008) evaluated the effect of Al and Mg on the specific rate of ferrous iron oxidation in Leptospirillumferriphilum and showed that increased salt concentrations increasingly depressed the specific rate of ferrous iron oxidation and also shifted the viable range increasingly towards the low potential region. Aluminium significantly reduces the amount of carbon biomass maintained in the reactor, whereas magnesium actually enhances it at low concentrations (Ojumu et al. 2008). Similarly, the effect of high concentrations of Na2SO4 on the growth rate of At. ferrooxidans has been investigated. Blight and Ralph, (2004), and Demergasso et al. (2005) described community changes related to increased sulphate concentrations (up to 150 g/L) when analysing samples from a low-grade Cu sulphide test heap in Chile. Inhibition was observed at sulphate concentrations in excess of 50 g/L.
Another major challenge faced by biomining companies, particularly those operating heaps in arid and semi-arid zones, is the quality of water used for irrigating the heaps. A high salt level is a significant problem in many areas but especially in the Western Australian and Chilean mineral processing industries. The growth and activity of biomining micro-organisms is significantly reduced in the presence of salt, particularly chloride ions (Zammit et al., 2009). The need to identify and characterize species and consortia of salt-tolerant mineral-oxidizing acidophiles has been recognized as a research priority and several papers referring to the isolation of salt-tolerant cultures with the ability to oxidize iron in the presence of NaCl have been published (Davis-Belmar et al., 2008, Zammit et al., 2009).
Microbial attachment to mineral surfaces
Attachment of micro-organisms to ore particles has been well proven and the attachment to metal sulphides and the formation of a biofilm is being viewed as critical for bioleaching performance (Sand et al., 1995). It is now generally recognized and accepted that bioleaching is mainly a chemical process where ferric iron and protons are responsible for the leaching reactions. The role of the microorganisms is to produce the leaching reagents and to create the space in which the leaching reactions take place. Microorganisms typically form an exopolysaccharide (EPS) layer when they adhere to the surface of a mineral. It is within this EPS layer rather than in the bulk solution that the oxidation reactions take place, as illustrated in Figure 1, and therefore the EPS serves as the reaction space (Rawlings, 2005).
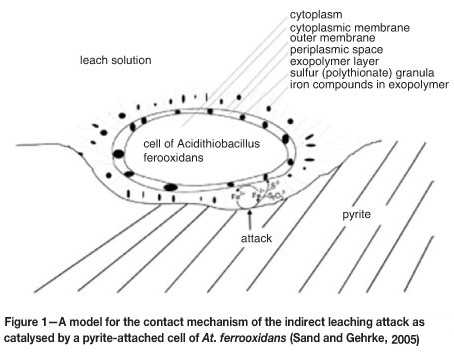
The importance of understanding microbial attachment was further demonstrated by Bouffard and Dixon (2002), who showed that the number of iron- and sulphur-oxidizing cells attached weakly and/or firmly onto ore particles far exceeds the number of planktonic cells. Thus, modelling the microbiology of the heap biooxidation process by considering solely the suspended microbial population would be deceptive.
In heap leach studies little attention has been paid to the investigation of microbial attachment as a means of mitigating heap bioleaching process difficulties. Aspects that could affect the performance of the microbes and the heap include initial microbial attachment to the ore, the development of firmly attached biofilms, the location of the microbial community with respect to the ore, and the kinetics of microbial growth on the ore surface and its subsequent impact on microbial ecology.
Recently the results from a number of studies investigating microbial attachment and colonization in heap bioleach environments were published. Africa et al. (2009) described the attachment of At. ferrooxidans and L. ferriphilum to four different mineral surfaces (pyrite and chalcopyrite concentrates, low grade run-of-mine ore, and quartz) using a 'particle-coated column reactor' and a 'biofilm reactor', representative of attachment dynamics in a heap.
In another study by Chiume et al. (2010) the impact of irrigation on microbial colonization was investigated. It was demonstrated that the rate at which microbes multiply and attach to ore was influenced by the irrigation rate. In particular, the enhancement of microbial surface colonization at lower irrigation rates was observed, as illustrated by the increase in attached and interstitial cell numbers with time, implying that the effect on colonization should be borne in mind when specifying irrigation rates.
Bromfield et al. (2010) studied the effect of temperature on the attachment of the thermophilic Metallosphaera hakonensis to a copper sulphide and confirmed that attachment of thermophilic archaea is suppressed at mesophilic temperatures. The data suggested that a secondary inoculation of thermophiles, once the heap has reached 40-45°C, may enhance their retention and improve subsequent colonization. These results support the recommendations on inoculation strategies made in a previous section of this paper.
Conclusions
The past two decades have witnessed exponential growth in the technological advancement and commercialization of heap bioleaching of copper ores, particularly of acid-soluble and secondary copper sulphides such as chalcocite. The treatment of low-grade chalcopyrite ores using heap bioleaching is currently the focus of numerous research efforts.
In the area of microbiology, the monitoring of microbial populations in heaps using a combination of molecular and culture-based techniques is now possible, and can provide an assessment of how microbial populations change in response to temperature and other heap conditions, especially important when treating chalcopyrite. Other aspects receiving attention include the mechanism of microbial attachment to mineral surfaces, the effect of process parameters such as irrigation rates on microbial colonization, the isolation and identification of salt-tolerant mineral-oxidizing acidophiles, and the effect of the build-up of elements such as sulphates, aluminium, and magnesium that could inhibit microbial performance.
The value of genetic studies probably lies in the indirect improvement of bioleaching through an enhanced understanding of the genetics and physiology of the organisms, rather than directly by the addition of genetically manipulated micro-organisms to commercial processes.
Several strategies for the inoculation of heaps have been described and the overall conclusion is that there would likely be a considerable time saving in inoculating new heaps with a microbial consortium (or consortia) at the appropriate points in time, rather than waiting for micro-organisms to grow naturally.
During the initial application of sulphide heap bioleaching, the performance was often poor because the microbial communities simply responded to the chemical and physical changes in the heap. The ability to now quantify and identify the microbes present in heap bioleach processes, and the growing understanding of the dynamics of these microbial communities and the effect of process conditions on their performance, facilitate the improved management and control of conditions in the heap, allowing successful microbial succession, better performance, and higher metal extractions.
References
AFRICA, C.-J., VAN HILLE, R.P., and HARRISON, S.T.L.. 2009. Investigation and visualisation of microbial attachment trends to sulphide minerals in a bioleach environment. Advanced Materials Research, vol. 71-73. pp 345-348. [ Links ]
BLIGHT, K.R. and RALPH, D.E. 2008. Aluminium sulphate and potassium nitrate effects on batch culture of iron oxidising bacteria. Hydrometallurgy, vol. 92. pp. 130-134. [ Links ]
BLIGHT, K.R. and RALPH, D.E 2004. Effect of ionic strength on iron oxidation with batch cultures of chemolithotrophic bacteria. Hydrometallurgy, vol. 73. pp. 325-334. [ Links ]
BOUFFARD, S.C. and DIXON, D.G.. 2002. On the rate-limiting steps of pyritic refractory gold ore heap leaching: results from small and large column tests. Minerals Engineering, vol. 15. pp. 859-870. [ Links ]
BRIERLEY, C. 2001. Bacterial succession in bioheap leaching. Hydrometallurgy, vol. 59. pp. 249-255. [ Links ]
BRIERLEY, C.L. 2008. How will biomining be applied in future? Transactions of the Nonferrous Metals Society of China, vol. 18. pp. 1302-1310. [ Links ]
BRIERLEY, J.A. and HILL, D.L. 1993. Biooxidation process for recovery of gold from heaps of low-grade sulfidic and carbonaceous sulfidic ore materials. US Patent 5,246,486. September 21. [ Links ]
BRIERLEY, J.A. and KUHN, M.C. 2010. Fluoride toxicity in a chalcocite bioleach heap process. Hydrometallurgy, vol. 104. pp. 410-413. [ Links ]
BROMFIELD, L., AFRICA, C,-J., HARRISON, S.T.L., and VAN HILLE, R.P. 2010 The effect of temperature on the attachment of Metallosphaera hakonensis to a copper sulphide concentrate with application to heap bioleaching. Bio and Hydrometallurgy '10, Cape Town, 8-9 November 2010. 23 pp. [ Links ]
CHUIME, R., MINNAAR, S., NGOMA, E., BRYAN, C., and HARRISON, S.T.L. 2010. Investigating microbial colonisation in bioheaps with varying irrigation rate. Bio and Hydrometallurgy '10, Cape Town, 8-9 November 2010. 19 pp. [ Links ]
CORAM-ULIANA, N.J., VAN HILLE, R.P., KOHR, W.J., and HARRISON, S.T.L. 2006. Development of a method to assay the microbial population in heap bioleaching operations. Hydrometallurgy, vol. 83. pp. 237-244. [ Links ]
CÓRDOBA, E.M., MUNÕZ, H.J.A., BLAZQUEZ, M.L., GONZALEZ, F., and BALLESTER, A. 2008. LEaching of chalcopyrite with ferric iron. Part I: general aspects. Hydrometallurgy, vol. 93. pp. 81-97. [ Links ]
DAVIS-BELMAR, C.S., NICOLLE, J.L.C., and NORRIS, P.R. 2008. Ferrous iron oxidation and leaching of copper ore with halotolerant bacteria in ore columns. Hydrometallurgy, vol. 94. pp.144-147. [ Links ]
DEMERGASSO, C., GALLEGUILLOS, P., ESCUDERO, L., ZEPEDA, V., CASTILLO, D., and CASAMAYOR, E. 2005. Molecular characterization of microbial populations in low-grade copper ore bioleaching test heap. Hydrometallurgy, vol. 80. pp. 241-253. [ Links ]
DEW, D., STEYN, J.W., and MINNAAR, S.H. 2009. High temperature heap leaching process. World Patent WO 2009/059 336. May 7. [ Links ]
DOPSON, M., BAKER-AUSTIN, C., KOPPINEEDI, P.R., and BOND, P.L. 2003. Growth in sulphidic mineral environments: metal resistance mechanisms in acidophilic micro-organisms. Microbiology, vol.149. pp. 1959-1970. [ Links ]
DOPSON, M., HALINEN, A-K., RAHUNEN, N., BOSTRÕM, D., SUNDKVIST, J-E., RIEKKOLA-VANHANEN, M., KAKSONEN, A.H., and PUHAKKA, J.A. 2008. Silicate mineral dissolution during heap bioleaching. Biotechnology and Bioengineering, vol. 99. pp. 811-820. [ Links ]
DU PLESSIS, C. 2003. Delivery system for heap bioleaching. World Patent WO 2,003,068,999. August 21. [ Links ]
DU PLESSIS, C.A., BATTY, J.D., and DEW, D.W. 2007. Commercial application of thermophile bioleaching. Biomining. Rawlings, D.E. and Johnson, B.D. (eds.). Springer-Verlag, Heidelberg, pp. 57-80. [ Links ]
GALLEGUILLOS, P.A., HALLBERG, K.B., and JOHNSON, D.B. 2009. Microbial diversity and genetic response to stress conditions of thermophilic bacteria isolated from the Escondida copper mine. Advanced Materials Research, vol. 7173. pp 55-58. [ Links ]
GERICKE, M., MULLER, H.H., NEALE, J.W., NORTON, A.E., and CRUNDWELL, F.K. 2005. Inoculation of heap-leaching operations. Proceedings of the 16th International Biohydrometallurgy Symposium 2005, Cape Town, South Africa. [ Links ]
GUNNERIUSSON, L., SANDSTRÕM, Â., HOLMGREN, A., KUZMANN, E., KOVACS, K., and VÉRTES, A. 2009. JAROSITE INClusion of fluoride and its potential significance to bioleaching of sulphide minerals. Hydrometallurgy, vol. 96. pp. 108-116. [ Links ]
HAWKES, R.B., FRANZMANN, P.D., AND PLUMB, J.J. 2006. Moderate thermophiles including 'Ferroplasma cupricutmlans' sp. nov. dominate an industrial-scale chalcocite heap bioleaching operation. Hydrometallurgy, vol. 83. pp. 229-236. [ Links ]
HUNTER, C.J. 2001. A bacterially assisted heap leach. World Patent WO 0144,519, 21. June 2001. [ Links ]
HUNTER, C.J. and Williams, T.L. 2002. Adaptation of bacteria for leaching, World Patent WO 02,066,689. August 29. [ Links ]
HUNTER, C.J. 2002. A method for the bacterially assisted heap leaching of chalcopyrite. World Patent 2002 70757 (A1) (US 2004 091984). [ Links ]
JOHNSON, D.B. and Hallberg, K.B. 2007. Techniques for detecting and identifying acidophilic mineral-oxidizing microorganisms. Biomining. Rawlings, D.E. and Johnson, D.B. (eds.). Springer-Verlag, Heidelberg. pp. 237-262. [ Links ]
JOHNSON, D.B. 2008. Biodiversity and interactions of acidophiles: Key to understanding and optimizing microbial processing of ores and concentrates. Transactions of the Nonferrous Metals Society of China, vol. 18. pp. 1367-1373. [ Links ]
KING, J.A. 2001. Method for initiating heap bioleaching of sulfidic ores. US Patent 6,207,443. March 27. [ Links ]
LOGAN, T.C., SEAL, T., and BRIERLEY, J.A. 2007. Whole-ore heap biooxidation of sulfidic gold-bearing ores. Biomining. Rawlings, D.E. and Johnson, B.D. (eds.). Springer-Verlag, Heidelberg.. pp. 113-138. [ Links ]
MORALES, P. and BADILLA, R. 2010. Process to increase the bioleaching speed of ores or concentrates of sulphide metal species by means of continuous inoculation with leaching solution that contains isolated microorganisms, with or without native microorganisms. US Patent 7837760 B2. 23 November 2010. [ Links ]
OJUMU, T.V., PETERSEN, J., and HANSFORD, G.S. 2008. The effect of dissolved cations on microbial ferrous-iron oxidation by Leptospirillumferriphilum in continuous culture. Hydrometallurgy, vol. 94. pp. 69-76. [ Links ]
OJUMU, T.V., Petersen, J., Searby, G.E., and Hansford, G.S. 2006. A review of rate equations proposed for microbial ferrous-iron oxidation with a view to application to heap bioleaching. Hydrometallurgy, vol. 83. pp. 21-28. [ Links ]
OKIBE, N. and JOHNSON, D.B. 2011. A rapid ATP-based method for determining active microbial populations in mineral leach liquors. Hydrometallurgy, vol. 108. pp. 195-198. [ Links ]
OKIBE, N., GERICKE, M., HALLBERG, K.B,. and JOHNSON, D.B. 2003. Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred tank bioleaching operation. Applied Environmental Microbiology, vol. 69. pp. 1936-1943. [ Links ]
QIN, W., ZHEN, S., YAN, Z., CAMPBELL, M., WANG, J., LIU, K., and ZHANG, Y. 2009. Heap bioleaching of a low-grade nickel-bearing sulphide ore containing high levels of magnesium as olivine, chlorite and antigorite. Hydrometallurgy, vol. 98. pp. 58-65. [ Links ]
RAWLINGS, D.E. 2005. Characteristics and adaptability of iron-and sulphur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microbial Cell Factories, vol. 4. pp.13. [ Links ]
RAWLINGS, D.E. 2011. Some important developments in biomining during the past thirty years. 19th International Biohydrometallurgy Symposium, Changsha, China, 18-22 September 2011. pp. 3-12. [ Links ]
RAWLINGS, D.E. and JOHNSON, D.B. 2007. The microbiology of biomining: development and optimization of mineral-oxidizing microbial consortia. Microbiology, vol. 153. pp.315-324. [ Links ]
REMONSELLEZ, F., GALLEGUILLOS, F., JANSE VAN RENSBURG, S., RAUTENBACH, G.F., GALLEGUILLOS, P., CASTILLO, D., and DEMERGASSO C. 2007. Monitoring the microbial community inhabiting a low-grade copper sulfide ore by quantitative real-time PCR analysis of 16S rRNA genes. Advanced Materials Research, vol. 20-21. pp. 539-542. [ Links ]
RENMAN, R., XINGYU, L., GANG, Z., JINGHE, C., JIANKANG, W., and DIANZUO. 2011. Industrial practice of a distinct bioleaching system operated at low pH, high ferric concentration, elevated temperature and low redox potential for secondary copper sulphide. Hydrometallurgy, vol. 108. pp. 130-135. [ Links ]
RIEKKOLA-VANHANEN, M. 2010. Talvivaara Sotkamo mine-bioleaching of a polymetallic nickel ore in subarctic climate. Nova Biotechnologica, vol. 10. pp.7-13. [ Links ]
SAND, W., GEHRKE, T., and HALLMANN, R. 1995. Sulfur chemistry, biofilm, and the (in) direct attack mechanism a critical evaluation of bacterial leaching. Applied Microbiology and Biotechnology, vol. 43. pp. 961-966. [ Links ]
SAND, W. and GEHRKE, T. 2005. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria. Research in Microbiology, vol. 157. pp. 49-56. [ Links ]
SUNDKVIST, J.E., SANDSTRÖM, Â., GUNNERIUSSON, L., and LINDSTRÖM E.B. 2005. FLUORINE toxicity in bioleaching systems. Proceedings of the 16th International Biohydrometallurgy Symposium 2005. Cape Town, South Africa. pp. 19-28. [ Links ]
VAN STADEN, P.J. 2008. Heap leach research at Mintek. ALTA 2008 Copper, Melbourne. ALTA Hydrometallurgical services. 13 pp. [ Links ]
WATLING, H.R. 2006. The bioleaching of sulphide minerals with emphasis on copper sulphides-A review. Hydrometallurgy, vol. 84. pp. 81-108. [ Links ]
WATLING, H.R., ELLIOT, A.D., PERROT F.A., and SHIERS D.W. 2009. Impacts of mineralogy on the chemistry and microbiology of heap bioleaching. Advanced Materials Research, vol. 71-73. pp 369-372. [ Links ]
XINGYU, L., BOWEI, C., JIANKANG, W., and RENMAN, R. 2010. Leptospirillum forms a minor portion of the population in Zijinshan commercial non-aeration copper bioleaching heap identified by 16S rRNA clone libraries and real-time PCR. Hydrometallurgy, vol. 104. pp. 399-403. [ Links ]
ZAMMIT, C.M., MUTCH, L.A., WATLING, H.R., and WATKIN, E.L.J. 2009. The characterization of salt tolerance in biomining microorganisms and the search for novel salt tolerant strains. Advanced Materials Research, vol. 71-73. pp. 283-286. [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2012. ISSN2225-6253. This paper was first presented at the Percolation Leaching Conference, 8-9 November 2011, Misty Hills, Muldersdrift.














