Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 no.10 Johannesburg Dez. 2012
PAPERS
Removal of unburned carbon from fly ash using a cyclonic-static microbubble flotation column
Y.J. CaoI, II; G.S. LiI; J.T. LiuI; H.J. ZhangI; X. ZhaiI
ISchool of Chemical Engineering and Technology, China University of Mining and Technology, China
IIChinese National Engineering Research Center of Coal Preparation and Purification, Xuzhou, China
SYNOPSIS
The purpose of this study was to investigate the flotation behaviour of unburned carbon in a cyclonic-static microbubble flotation column (FCSMC). The ash sample, collected from a power station in Guangdong province of China, was characterized by size analysis, X-ray diffraction, contact angle measurements, and X-ray fluorescence. The effect of the column flotation operating variables on the removal of unburned carbon from the fly ash was systematically studied. The feasibility of separating unburned carbon and ash was determined from the removal rate of unburned carbon (RUC) and loss on ignition (LOI). Within the range studied, the optimum diesel oil dosage was 1200 g/t, abies oil dosage was 600 g/t, pulp density was 20 per cent, superficial gas velocity was 1.4 cm/s, and circulating pressure was 0.20 MPa. The results indicate that the FCSMC technique is effective in removing the unburned carbon from the fly ash, which can be attributed to the generation of microbubbles and the continuous cyclonic circulation method. Under the optimized conditions, a cleaning ash with 2.13 per cent LOI and 94.21 per cent RUC was obtained.
Keywords: unburned carbon, coal fly ash, column flotation.
Introduction
Fly ash is the main by-product of coal combustion. It is a solid, fine, and powdery material that consists of mainly non-combustible inorganic materials and unburned carbons. In 2009 over 375 Mt of fly ash, resulting from burning coal at power stations, was produced in China1. Pollution issues associated with fly ash are attracting more and more attention. However, utilization remains limited due to some technical limitations. The key concern is the high unburned carbon content in fly ash. For example, for use as a raw material in cement and concrete, the unburned carbon in fly ash should be less than 3 per cent. The unburned carbon would be removed by 'dry' or 'wet' processes like electrostatic separation, gravitational separation, or froth flotation. Various studies have been carried out on the recovery of combustibles from ash materials2-5. Studies showed that froth flotation is the most efficient method for the removal of unburned carbon, compared to electrostatic or other physical cleaning techniques6,7.
Column flotation is an important method in mineral processing technology. It is regarded to have reached maturity and offers some advantages over conventional flotation. The advantages of column flotation include a smaller physical footprint, lower power requirement, and a greater ability to recover valuable fines at a better grade of target mineral due to the minimization or prevention of hydraulic entrainment of undesirable fines8-10.
The introduction of the cyclonic circulation method is one of the most important developments in column flotation technology in the past few years. Research by Yalcin11 indicated that centrifugal force field in the flotation device is able to provide a high rate of bubble-particle contact and enables the process to take place in a very short column. FCSMC technology, in which column flotation and centrifugal force field are combined, is a novel method developed in recent years at China University of Mining and Technology, and related studies indicate that it has some advantages over conventional flotation machines12-15.
The FCSMC separator can be divided into three main zones, as described in Figure 1, i.e. the column separation zone, the cyclonic separation zone, and pipe flow mineralized zone. The column separation zone is where hydrophobic particles (concentrate particles) adhere to bubbles by the way of counter current collision and bubbles disengage from the pulp. This zone is in the upper part of the column. The cyclonic separation zone is in the form of an inverted cone, in which a centrifugal force field is generated when circulating pulp is injected tangentially into the column. In the cyclonic separation zone, the circulating materials are split into three fractions due to the centrifugal force. The particles with high density do not adhere to the bubbles, and move towards the column wall and then downward along the column wall; the mineralized bubbles, which include a substantial amount of valuable minerals, move towards the column center and then upward to the column separation zone; the remainder are pumped by the circulation pump as the middlings. In the pipe flow mineralized zone, a self-aerating bubble generator in which air is drawn in by the negative pressure induced by jet flow is used to generate microbubbles. Due to a high mixing velocity and a large interfacial area in the pipe flow mineralized zone, there is rapid contact and collection of particles. Compared to mechanical cells and other flotation columns, conceptually, the FCSMC separator is characterized by the following three unique advantages16-18:
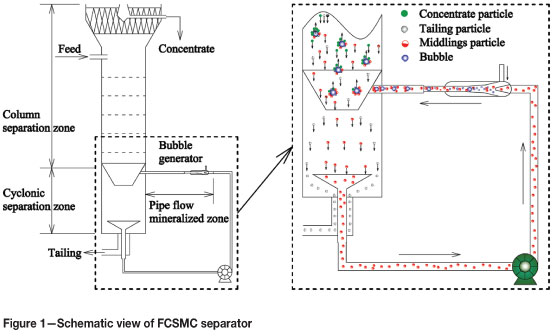
Microbubbles generated by the self-aerating bubble generator are able to enhance the recovery of fine particles
Continuous circulation of the pulp containing the middlings strengthens the recovering capacity of the column
The cyclonic flotation method employed in the column reinforces mineralization efficiency and reduces the lower limit for efficient flotation by increasing circulation pressure.
Experimental
Materials
Fly ash from a power station located in Guangdong province was used for this study. An 80 kg portion of a representative sample was split and bagged in 4 kg sub-samples to keep them as homogeneous as possible. The unburned carbon in the fly ash and the flotation products was evaluated by loss on ignition (LOI) analysis. LOI analyses were done with moisture-free fly ash samples dried in a laboratory furnace. After drying, i g of representative sample was put into a ceramic crucible and placed in a laboratory furnace. The sample was kept at 815°C for 3 hours. The residue was then allowed to cool in a desiccator and weighed. LOI% was calculated using the formula:

Mineralógica! and chemical analysis of the sample
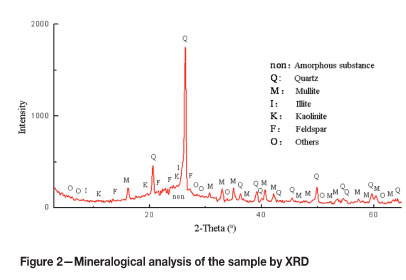
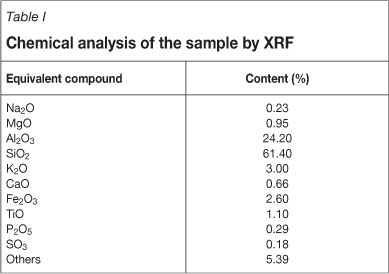
X-ray diffraction (XRD) analysis was carried out using a D/Max-3B (Rigaku) unit. The results are shown in Figure 2. Chemical analysis of the sample was carried out using an X-ray fluorescence (XRF) unit (S8 TIGER), and the results obtained are reported in Table I.
Contact angle measurements
Contact angle has been extensively used in mineral processing theory and practice. For a bubble to actually adhere to mineral particle so that the latter can be recovered, a finite contact angle must exist at the three-phase line of contact. Contact angle is not the sole criterion responsible for mineral recovery, but is one of the important contributing factors19.
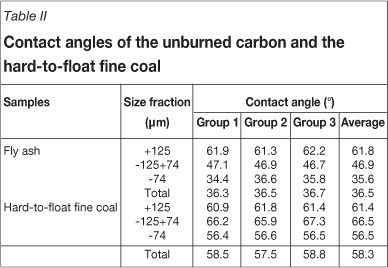
In this study, the unburned carbon was collected by sieving the ash sample using a Tyler screen with sieve diameter of 74 µn. Samples with the size fraction larger than 74 µm were used as the unburned carbon for contact angle measurements. LOI of the unburned carbon obtained using this method was about 75 per cent. Contact angles of the samples were determined by the sessile drop method using a digital goniometer (Drop Shape Analysis System, DSA100, Krüss GmbH, Hamburg, Germany). For contact angle measurements, a drop of the test liquid was dispensed at a constant rate by a microsyringe steel needle. The drop shape was monitored with a digital camera for 20 seconds, and contact angle, drop diameter, and volume were recorded. The contact angle was calculated by the Young-Laplace method. In order to direct the problem description, the contact angles of a hard-to-float fine coal from a coal preparation plant in Hebei province were also measured. The measurements were repeated three times for every sample. The results of the contact angle measurements are shown in Table II.
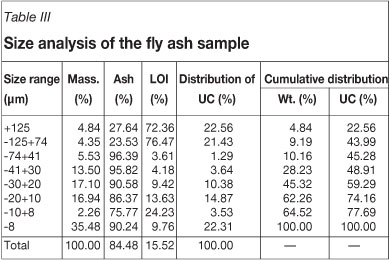
Size analysis of the sample
A 150 g portion of the ash sample was sieved using the cyclonic particle analyser and various size fractions collected for analysis. All the sieved samples were dried in an oven at about 100°C and cooled to room temperature. Each size fraction was analysed for the unburned carbon (UC) content, i.e. LOI%. The results are given in Table III.
Experimental setup and tests
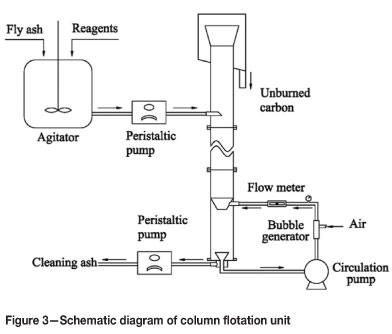
The column flotation unit
A laboratory-size column flotation unit was designed for the experiments. The unit consists of an agitator, a peristaltic pump for feeding, a circulation pump, a tailings peristaltic pump, and the column body. The column was made of Plexiglas of 100 mm in diameter and 2000 mm in height. The self-adsorption microbubble generator was used to generate microbubbles. A schematic of the column flotation unit is shown in Figure 3.
Experimental procedure and conditions
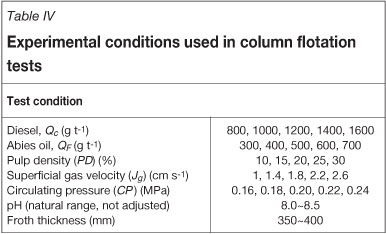
For the flotation process, slurry with a certain pulp density (solids content by weight) was formed and agitated in the agitator for 5 minutes. Collector and frother were then added. Conditioning between the additions of each reagent was allowed. Conditioning times were 3 minutes for the collector and 1 minute for the frother. The feed to the column was introduced from the upper section of the collection zone by pumping the slurry from the agitator. Wash water for cleaning of the froth product was introduced through a perforated plastic container situated just above the froth zone. The circulation pressure of the flotation column was metered by a pressure gauge and controlled by the circulation pump. The superficial gas velocity was metered by a flow meter. The test conditions and reagents used are presented in Table IV. Flotation products were filtered, dried, and analysed for unburned carbon contents (LOI, %).
Results and discussion
Characteristics of the sample
The XRD results reported in Figure 2 indicate that most of the materials in the ash sample are amorphous. The crystalline minerals in the ash sample contain mainly quartz (Q), mullite (M), illite (I), kaolinite (K), and feldspar (F). Mullite is an alumininosilicate material (3Al2O3-2SiO2) that forms at high temperatures by an interaction of silica and alumina-bearing minerals in the combustion environment. Table I shows the chemical components of the sample. As can be seen, SiO2 and Al2O3 were the major components in the fly ash. According to characterization by ASTM C 618, a fly ash sample having a SiO2 + Al2O3 + Fe2O3 content greater than 70 per cent and CaO less than 10 per cent belongs to the F class20.The ash used in this study can therefore be classified as F class fly ash.
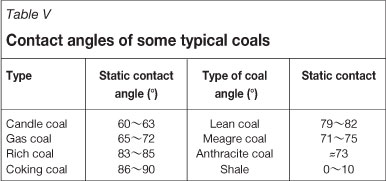
Results of contact angle measurements of the unburned carbon and the hard-to-float fine coal are listed in Table II. It can be seen that, in the +125 µm fraction, the contact angle of the unburned carbon is nearly equal to that of the hard-to-float fine coal. Contact angles of the two samples all decrease with decreasing particle size. However, the contact angles of the unburned carbon in the -125 µm +74 µm and -74 µm fractions are obviously lower than that of the hard-to-float coal. The contact angle for the entire sample of the unburned carbon sample is 36.5° which is 21.8° less than that of the hard-to-float fine coal. Previous study of flotation of the hard-to-float fine coal showed that the cleaning of this kind of coal presents some challenges to be overcome21. This phenomenon indicated that the removal of the unburned carbon from fly ash using flotation is more difficult, due to the lower contact angle. In addition, the authors also cited the contact angles of some typical coals (listed in Table V) as a comparison22. As shown, the contact angles of nearly all the coal types are well above that of unburned carbon (Table II). This indicates that the unburned carbon contained in fly ash has a lower hydrophobicity, and these results correlate well with the study by Demir20.
In China, fly ash used in concrete and cement applications is required to have a LOI content generally less than 5 per cent by weight in order to obtain the required pozzolanic properties. This requirement results from the fact that properties of concrete incorporating high-carbon ash are inferior to those of concrete incorporating low-carbon ash. For the fly ash used in this study, the size analysis results given in Table III show that more than 54 per cent of the fly ash was in slime form (-20 µrn), where about 40 per cent of the unburned carbon was located. In the coarse fraction (+74 µm), the LOI was 74.31 per cent. Although this fraction had the lower weight distribution (9.19 per cent), it contained more than 43 per cent of the unburned carbon. From the above analysis, it is obvious that the removal of the unburned carbon distributed in +74 µm and -20 µm fractions is the key to reducing the LOI of the ash sample.
Column flotation tests
The experimental conditions and range of variables used in the column flotation tests are summarized in Table IV. The effect of operating parameters on flotation results was evaluated by the removal rate of unburned carbon and LOI of the cleaning ash.
Influence of collector dosage
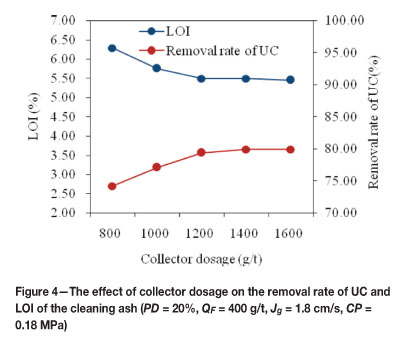
The collector dosage is a critical parameter in the flotation experiments. Adjusting the collector dosage can change the surface character of the particles and influence the recovery efficiency. Generally, unburned coal samples from power stations are severely oxidized to the extent of losing about 55 per cent of their aliphatic hydrocarbon groups, and this results in the hydrophobicity of unburned carbon being lower than that of the coal before combustion. Thus, the dosage of collector is generally at high levels. Diesel oil was chosen as collector in the trials. Five different collector dosages (800, 1000, 1200, 1400, and 1600 g/t) were used in this study. The effect of collector dosage on the removal rate of unburned carbon (RUC) and LOI of the cleaning ash is shown in Figure 4, where it is seen that 1200 g/t collector dosage resulted in a RUC of 79.38 per cent and a LOI of 5.49 per cent. When the dosage of collector is over 1200 g/t, no further increase in RUC and no further decrease in LOI were observed. Therefore, 1200 g/t was determined as the optimum collector dosage.
Influence of frother dosage
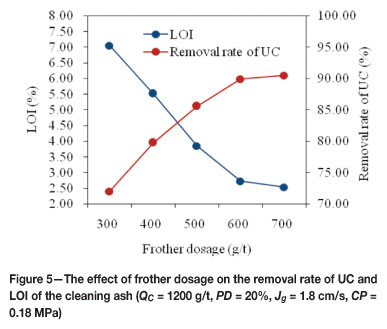
The stability of the bubble-particle aggregate is one of the prerequisites for a successful flotation operation. The frother concentration directly affects the bubble size (by controlling bubble coalescence), selectivity, and recovery. In order to determine the effect of the frother concentration on the column flotation performance, abies oil was used as frother. Figure 5 shows the results obtained with abies oil dosages ranging from 300 g/t to 700 g/t. The removal rate of UC increased from about 72 per cent to 90 per cent as the dosage of frother was increased from 300 g/t to 600 g/t. LOI of the cleaning ash showed a decrease with increased frother dosage. However, the removal rate of both UC and LOI varied very slightly variation when the frother dosage was over 600 g/t. Therefore, 600 g/t was determined as the optimum collector dosage.
Influence of pulp density
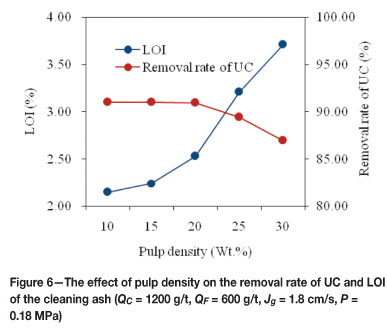
It is well known that pulp density is an important parameter in flotation. The selection of flotation pulp density is determined by the recovering capacity, selectivity, and froth load capacity for valuable particles. Generally, the higher the yield of froth phase, the lower the required pulp density. For example, the pulp density of coal flotation is arranged at a lower level about 100 g/L (10 wt.%) or so due to the higher yield of froth phase (60 per cent to approximately 80 per cent). In this study, the froth phase yield is about 30 per cent to 40 per cent, which is much lower than that of coal flotation. Therefore, pulp densities used in fly ash flotation are generally higher than those used in fine coal flotation. The column flotation tests in this study were carried out at different pulp densities (10-30 per cent) in the FCSMC separator in order to determine the effect on flotation performance of the fly ash. The results of these tests are plotted in Figure 6. As can be seen, when the pulp density increased from 10 per cent to 20 per cent, the RUC (percentage) showed a slight change. Increasing the pulp density, however, brought about a significant decrease in the RUC of up to 30 per cent. From Figure 6, it is also obvious that the LOI of the cleaning ash increased from 2.15 per cent to 3.71 per cent as the pulp density was increased from 10 per cent to 30 per cent. A pulp density of 20 per cent gave a LOI value of 2.53 per cent, which met the requirements that LOI value should be lower than 3.0 per cent. Taking into account the flotation column capacity, 20 per cent was determined as the optimum pulp density.
Influence of superficial gas velocity
The superficial gas velocity is the ratio between volumetric gas flow rate rate and the cross-sectional area of the column cell. It is a parameter related to the bubble size, and has a direct influence on the flotation recovery. The effect of superficial gas velocity was investigated in the range from 1.0 to 2.6 cm/s. The results are presented in Figure 7. When the superficial gas velocity was at a low level of 1.0 cm/s, a large proportion of unburned carbon was not floated. The RUC and LOI of the cleaning ash were 3.13 per cent and
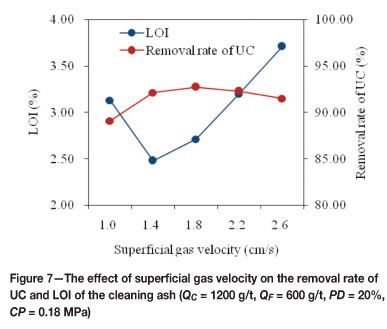
89.07 per cent, respectively. Increasing the superficial gas velocity from 1.4 to 2.6 cm/s resulted in the LOI of the cleaning ash increasing almost linearly, while the RUC showed a slight change. The increasing of LOI with increasing superficial gas velocity may be due to the deterioration in the separation environmental resulting from the increase in turbulent flow. Further experiments were carried out at 1.4 cm/s in view of the above analysis.
Influence of circulating pressure
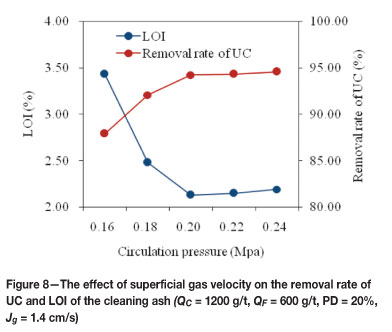
The pulp circulation is the typical characteristic of FCSMC separator. In the circulation process, the jet of the circulation pulp entrains air. Due to a high mixing velocity and a large interfacial area, there is rapid contact and collection of air bubbles/particles. Previous studies demonstrated that an increase in circulating pressure was able to reduce the lower limit for efficient flotation and enhance collection16,17. The circulating pressure was varied from 0.16 to 0.24 MPa. The results, which are shown in Figure 8, show that the removal rate of UC increased from about 87.89 per cent to 94.21 per cent as the circulating pressure was increased from 0.16 MPa to 0.20 MPa. The LOI of the cleaning ash showed a decrease with increasing circulating pressure. This phenomenon can be explained by the fact that the mineralization efficiency and recovery capacity of the column were enhanced by increasing the circulating pressure. However, both the removal rate of UC and LOI showed a very slight variation when the circulating pressure was over 0.20 MPa. Therefore, 0.20 MPa was determined as the optimum circulating pressure.
Conclusions
The following major conclusions can be drawn from this study:
1. By optimizing the flotation operating parameters at collector dosage (diesel oil) of 1200 g/t, frother dosage (abies oil) of 600 g/t, pulp density of 20 per cent, superficial gas velocity of 1.4 cm/s, and circulating pressure of 0.20 MPa, UC has been successfully removed from the fly ash. The LOI (%) of the cleaning ash was decreased to 2.13 per cent,, while the removal rate of UC was 94.21 per cent.
2. The main advantages of the FCSMC separator are the generation of microbubbles and the continuous cyclonic circulation method. As a novel flotation column, the unique advantages make the FCSMC separator more efficient in reinforcing the mineralization efficiency and recovery of fine particles. In conclusion, the FCSMC separator seems to constitute a viable and effective separation process for removing unburned carbon from coal fly ash.
Acknowledgements
This work was performed under the auspices of the National Key Basic Research Program of China (2012CB214905), the National Nature Science Foundation of China (51074157, 51004107), and the Program for Postgraduates Research Innovation in Universities of Jiangsu Province (Project no. CXZZ11_0312).
References
1. Mao, S.D., Li, Z., and Fang, Y. Current status of research on the utilization of fly ash. Concrete, vol. 23, no. 7, 2011. pp. 82-84. (in Chinese) [ Links ]
2. Niewiadomski, M., Hupka, J., Bokotko, R., and Miller, J.D. Recovery of coke fines from fly ash by air sparged hydrocyclone flotation. Fuel, vol. 78, no. 2, 1999. pp. 161-168. [ Links ]
3. Huang, Y., Takaoka, M., and Takeda, N. Removal of unburned carbon from municipal solid waste fly ash by column flotation. waste Management, vol. 23 no. 4, 2003. pp. 307-313. [ Links ]
4. Emre Altun, E., Xiao, C., and Hwang, J.Y. Separation of unburned carbon from fly ash using a concurrent flotation column. Fuel Processing Technology, vol. 90, no. 12, 2009. pp. 1464-1470. [ Links ]
5. Ucurum, M. Influences of Jameson flotation operation variables on the kinetics and recovery of unburned carbon. Powder Technology, vol. 191, no. 3, 2009. pp. 240-246. [ Links ]
6. Hwang, J.Y. Wet process for fly ash beneficiation. PCT Patent Application No. US 1993/5047145. [ Links ]
7. Mcmahan, L.G., Kenneth, J.C., Yee, S., Richard, P.K., Mercedes, M.V., John, M.A., Michael, V.C., and Paul, H.Z. Physical cleaning of high carbon fly ash. Fuel Processing Technology, vol. 76, no. 1, 2002. pp. 11-21. [ Links ]
8. Finch, J.A. Column flotation: A selected review part IV: Novel flotation devices. Minerals Engineering, vol. 8, no. 6, 1995. pp. 587-602. [ Links ]
9. Rubio, J. Modified column flotation of mineral particles. International Journal of Mineral Processing, vol. 48, no. 3-4, 1996. pp.183-196. [ Links ]
10. Ityokumbul, M.T. Effect of pulp cleaning zone on gangue control in column flotation. Minerals Engineering, vol. 8, no. 10, 1995. pp.1231-1237. [ Links ]
11. Yalcin, T. The effect of some design and operating parameters in the cyclo-column cell. Minerals Engineering, vol. 8, no. 3, 1995. pp. 311-319. [ Links ]
12. Liu, J.T., Li, X.B., Wang, Y.T., Cao, Y.J., and Lü, F.K. Experimental study on separating some molybdenum ore by using cyclonic-static micro-bubble flotation column. Journal of Central South University (Science and Technology), vol. 39, no. 2, 2008. pp. 300-306. (in Chinese) [ Links ]
13. Zhou, C.C., Liu, J.T., Huang, G., Yan, B., and Ren, X.C. Study of bauxite flotation desilication by using flotation column. Journal of Central South University (Science and Technology), vol. 43, no. 3, 2010. pp. 846-851. (in Chinese) [ Links ]
14. Cao, Y.J., Gui, X.H., Ma, Z.L., Yu, X.X., Chen, X.D., and Zhang, X.P. Process mineralogy of copper-nickel sulphide flotation by a cyclonic-static micro-bubble flotation column. Mining Science and Technology, vol. 19, no. 6, 2009. pp. 784-787. [ Links ]
15. Li, L., Liu, J.T., Wang, Y.T., Cao, Y.J., Zhang, H.J., and Yu, H.S. Experimental research on anionic reverse flotation of hematite with a flotation column. Procedia Earth and Planetary Science, vol. 1, no. 1, 2009. pp. 791-798. [ Links ]
16. Li, G.S., Liu, J.T., Cao, Y.J., and Wang, D.P. Effect of a cyclonic flotation column on the separation of magnesium from phosphate ore. Mining Science and Technology (China), vol. 21, no. 5, 2011. pp. 647-650. [ Links ]
17. Zhou, X.H. and Liu, J.T. Particle residence time in column flotation based on cyclonic separation. Journal of China University of Mining & Technology, vol. 17, no. 3, 2007. pp. 349-353. [ Links ]
18. Liu, J.T. Cyclonic-static micro-bubble flotation apparatus and method. PCT Patent Application No. uS 1999/0232992. [ Links ]
19. Laskowski, J.S. and Ralston, J. Colloid chemistry in mineral processing, Elsevier, New York. 1992. [ Links ]
20. Demir, U., Yamik, A., Kelebek, S., Oteyaka, B., Ucar, A., and Sahbaz, O. Characterization and column flotation of bottom ashes from Tuncbilek power plant. Fuel, vol. 87, no. 6, 2008. pp. 666-672. [ Links ]
21. Tao, X.X., Cao, Y.J., Liu J., Shi, K.Y., Liu, J.Y., and Fan, M.M. Studies on characteristics and flotation of a hard-to-float high-ash fine coal. Procedia Earth and Planetary Science, vol. 1, no. 1, 2009. pp. 799-806. [ Links ]
22. Xie, G.Y. Mineral Processing. China University of Mining and Technology Press, Xuzhou. 2001. (in Chinese) ♦ [ Links ]
Paper received Mar. 2012; revised paper received Apr. 2012.
©The Southern African Institute of Mining and Metallurgy, 2012. ISSN2225-6253.














