Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 n.10 Johannesburg Dec. 2012
PAPERS
Calcination characteristics of laterite ores from the central region of Anatolia
E. KeskinkilicI; S. PournaderiII; A. GeveciII; Y.A. TopkayaII
IDepartment of Metallurgical and Materials Engineering of Atilim University, Ankara, Turkey
IIDepartment of Metallurgical and Materials Engineering of Middle East Technical University (METU), Ankara, Turkey
SYNOPSIS
Drying, calcination, prereduction, and smelting are the main steps in conventional crude ferronickel production. Industrially, these steps are conducted using the rotary kiln-electric arc furnace (RKEF) process. In this paper, calcination characteristics of Sivrihisar laterite ores from the Central Anatolia region are investigated. The extent of elimination of chemically bound water and other volatiles was studied by experiments conducted at various temperatures in the 250-800°C range. Phase changes were examined using X-ray diffractometry. For the particle size used in the study, 300°C was determined to be almost sufficient for complete transformation of goethite to haematite, and 700°C was required for effective elimination of all volatiles in the ore.
Keywords: laterite, calcination, nickel.
Introduction
Nickel is one of the important alloying elements for primary and secondary steelmaking. Approximately two-thirds of nickel produced in the world is used in stainless steelmaking1. As far as industrial nickel production is concerned, both hydrometallurgical and pyrometallurgical techniques are currently applied. There are three important nickel reserves in Turkey. Two of them are in the West Anatolia region; namely the Gordes and Caldag deposits. A number of studies have been carried out on nickel extraction from the Gordes and Caldag ores2-8. The third recently-discovered area containing nickel reserves is Sivrihisar, a town in Eskisehir located in the Central Anatolia region. During the first decade of the millennium, considerable reserves of oxidetype nickel ores have been found in the Yunusemre and Mihaliccik areas of Sivrihisar. Since then, mining facilities have been expanded to the Yunusemre Karasivritepe and Kucuksivritepe peaks. Sivrihisar laterite ore is limonitic in nature, with a high iron content and low MgO. The low arsenic content of the ore makes ferronickel smelting a suitable method for nickel extraction. Chemical analyses of the representative samples taken from the Yunusemre Karasivritepe and Kucuksivritepe locations are illustrated in Table I.

A project was started at the beginning of 2010 for ferronickel production from Sivrihisar laterite ores, supported by the Scientific and Technological Research Council of Turkey (TUBITAK). This laboratory-scale project involves the calcination, prereduction, and smelting stages of ferronickel production. This paper investigates the calcination characteristics of Sivrihisar laterite ores. The results of calcination experiments conducted in the scope of this work provide useful information regarding the calcination behavior of Sivrihisar laterites, which will hopefully be treated in the ferronickel plant in the Yunusemre region within the present decade.
Experimental
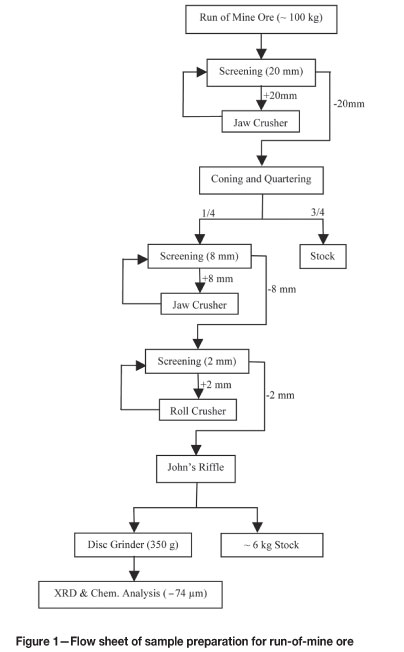
Sivrihisar limonitic laterite ore from the Yunusemre Karasivritepe and Kucuksivritepe peaks was used in the experiments. Four ore samples were taken from specific regions of the mine and were thoroughly mixed to form a representative sample for the experiments. The mixed sample was then divided into four almost equal weights of approximately 100 kg by the coning and quartering method. One quarter was taken for ore characterization and the others were stocked in barrels. The complete procedure for obtaining a representative sample from a run of mine ore for X-ray diffractometry (XRD) and chemical analysis is shown in Figure 1.
The XRD pattern of the run-of-mine ore is shown in Figure 2. XRD was carried out using a Rigaku SA-HF3 diffractometer with Cu Ka (k = 1.54 A) radiation (40 kV and 40 mA). The ore consists mainly of quartz (SiO2) with characteristic peaks at d101 = 3.33 A, d100 = 4.24 A, and d112 = 1.82 A; and goethite (FeO[OH]) with characteristic peaks at d110 = 4.17 A, d111 = 2.45 A, and d130 = 2.69 A. There are considerable amounts of calcite (CaCO3) with a characteristic peak at d104 = 3.01 A and haematite (Fe2O3), indicated by the characteristic peaks at d104 = 2.69 A, d110 = 2.51 A, and d116 = 1.69 A. Moreover, two low-intensity peaks were recorded at a low degree region (26 < 15° ): it was suggested that the peak at d = 16.59 A (26 = 5.32° ) is associated with nontronite, while the peak at d = 7.07 A (26 = 12.50° ) is the characteristic peak of kaolinite (Al2Si2O5 (OH)4).
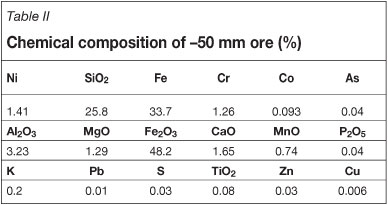
In the calcination experiments, it was determined that a particle size finer than 50 mm was to be used. Therefore, one of the three subsamples stocked in the barrels was screened at 50 mm. The oversize lumpy materials containing chiefly gangue minerals were rejected, and the particles passing 50 mm were labelled as '-50 mm ore'. A procedure similar to the one given in Figure 1 was applied for the -50 mm ore, and the particle size was reduced to below 1 mm by successive crushing and grinding steps. Samples were prepared for XRD analysis and chemical composition determination using a disc grinder. The chemical analysis of -50 mm ore is illustrated in Table II. The chemical analysis was conducted by ICP-OES.
An externally controlled muffle furnace was used in the calcination experiments. In each run, 100 g of laterite ore (-1 mm size) were placed in a chamotte tray and charged to the furnace (kept at the experimental temperature). Runs were carried out with temperature and time as the experimental variables. Two different approaches were used. In the first, calcination was continued until the charge weight became constantthese runs are termed 'continuous to constant weight'. In the second, calcination was carried out for a specified time in order to evaluate the difference in the weight in a noncontinuous way. In the continuous to constant weight runs, the tray was removed from the furnace periodically to record the weight, and was recharged immediately after weighing. In the second set of experiments, the tray was left in the furnace for a predetermined period. In both approaches, the furnace wall was opened at specified times to mix the charge.
With the first approach, experiments were conducted at 250°C, 300°C, 350°C, 400°C, 500 °C, 600°C, 650°C, 675°C, 700°C, and 800°C until the weight became constant. In the experiments conducted by the second approach, which can be regarded as the reproduction of the first approach, the weight loss at the end of specific times was determined.
Results and discussion
Thermogravimetric (TG) measurements were conducted in a thermobalance with DTA (differential thermal analysis) capability in the Central Laboratory of the Middle East Technical University. DTA-TG curves were obtained by heating approximately 20 mg of ground sample (< 74 µm) from 25°C to 1000°C with a heating rate of 10°C per minute. The DTA and TG behaviour of the -50 mm ore are illustrated in Figure 3.
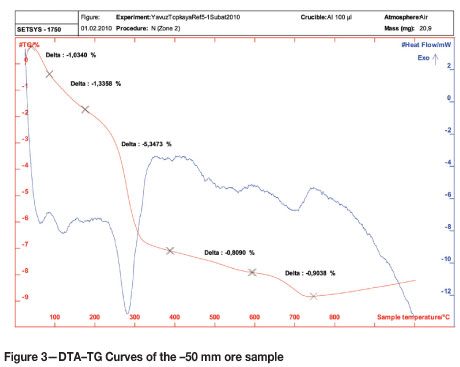
Five main endothermic peaks were observed at around 60°C, 110°C, 290°C, 500°C, and 700°C with a mass loss of about 1 per cent, 1.3 per cent, 5.3 per cent, 0.8 per cent, and 0.9 per cent respectively. Peaks at 60°C and 110°C correspond to evaporation of free moisture or physically bound water. The endothermic peak at about 290°C corresponds to the dehydroxylation of goethite, i.e. the transformation of goethite to haematite. As stated in the literature, this transformation has been subjected to a number of investigations and there has been no general agreement on the mechanism9. According to the studies carried out by Walter et al.10 and Watari et al.11, the transformation is suggested to take place directly according to reaction [1]:
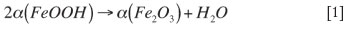
On the other hand, some researchers12-13 propose that an intermediate phase, known as proto-haematite or hydro haematite, forms before the final formation of haematite . In Figure 3, the peaks around 500°C and 700°C most likely correspond to the last stages of elimination of chemically bound water and carbon dioxide from the ore.
Considering the results of the TG measurements, it was decided to conduct the first experiment at 250°C. The run conducted at this temperature resulted in a weight loss of 5.9 per cent, while the time elapsed to reach constant weight was 120 minutes. The variation of weight loss values with time is illustrated in Figure 4. The experiment carried out at 300°C reached steady state at the end of 65 minutes, which was substantially less than the period necessitated at 250°C. The weight loss due to dehydroxylation was found to be 6.6 per cent. Therefore, it was concluded that 250°C was not sufficient to perform effective dehydroxylation for the particle size used in this work.
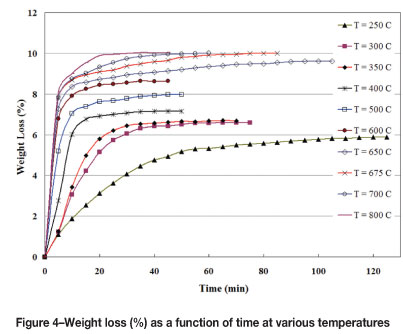
An increased weight loss was noted when the temperature was raised to 400°C. The run conducted at this temperature resulted in a weight loss value of 7.2 per cent. The further experiment at 500°C produced a weight loss of 8.0 per cent. In both runs, the charges reached constant weight at the end of 40-45 minutes. These findings obviously show that certain reaction(s) was/were taking place after the dehydroxylation of goethite, confirming the data obtained from the DTA-TG curves.
In order to continue investigating the extent of change in weight loss and the time required to reach constant weight, it was decided that experiments should be conducted at higher temperatures. The run carried out at 600°C gave rise to a weight loss of 8.6 per cent. The time elapsed to reach steady state was 40 minutes at this temperature. A higher weight loss value was reported at 700°C, at 10.0 per cent, as expected. On the other hand, the time needed for complete calcination was found to be 60 minutes, which was 50 per cent longer than that obtained at 600°C. This increase in calcination time was attributed to certain new reaction(s) taking place in the 600-700°C interval.
The experiment conducted at 800°C resulted in a weight loss of 10.0 per cent, which was almost the same value obtained at 700°C. Therefore, it was inferred that 700°C is sufficient for calcination and that chemically bound water and other volatiles in the ore are completely eliminated at a temperature less than or equal to 700°C. At 800°C, the time to reach constant weight was found to be 45 minutes, which was approximately 25 per cent shorter than the period at 700°C. This result was in accordance with expectations, since an increase in temperature is effective in increasing the rate of the calcination process.
To determine precisely the temperature at which the goethite-to-haematite transformation is completed, it was decided that a further experiment should be carried out at the mid-point of the 300-400°C interval. The run conducted at 350°C produced a weight loss of 6.7 per cent, which was nearly the same as that obtained at 300°C. This finding revealed that for the particle size of the ore used in the present study, 300°C was almost sufficient to achieve dehydroxylation. The time to reach steady state was 60 minutes, which is slightly shorter than that of the run conducted at 300°C, as expected.
As mentioned previously, approximately the same weight loss values were obtained at the end of the runs carried out at 700°C and 800°C. This result clearly indicates that removal of chemically bound water and other volatiles is completed at a temperature less than or equal to 700°C. In order to determine precisely this minimum temperature, an experiment was conducted at the mid-point of the 600-700°C range. The run carried out at 650°C resulted in a weight loss value of 9.6 per cent. This value is less than that obtained at 700°C, indicating that 650°C is not sufficient to remove all volatiles. The time to reach constant weight was 95 minutes, substantially longer than the period required at 700°C. To verify the results, the experiment was repeated and the same results were obtained. This rather long time is probably an indication of certain reversible reaction(s) at these temperatures, which increase the time necessary for the process to reach steady state. A further experiment at the mid-point of 650-700°C was carried out. At the end of the run conducted at 675°C, a weight loss of 10.0 per cent was recorded. This value is almost the same as the one obtained at 700°C. The time elapsed to reach constant weight was recorded as 75 minutes, which is slightly lower than the time required at 700°C. Therefore, it was concluded that 675°C is nearly high enough to conduct the calcination of -50 mm Sivrihisar laterite if sufficient time is allocated.
In summary, thermal experiments carried out in the range of 250-800°C showed that the amount of chemically bound water and other volatiles in the ore is approximately 10 per cent by weight. Roughly speaking, two-thirds of the weight loss is associated with the removal of chemically bound water during the goethite-haematite transformation, and the remaining one-third is related to the elimination of volatiles in the 350-700°C interval. The change in weight loss (%) with temperature is illustrated in Figure 5. In this figure, the time to reach constant weight is also shown for each temperature. Together with the findings described in the preceding paragraphs, the total process can be divided into three steps:
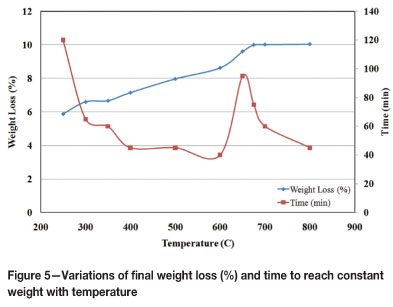
1. Removal of chemically bound water in the 0-350°C range
2. Reaction(s) in the 350-600°C interval: the shortest time was obtained at 600°C, indicating that this could be regarded as the termination point of the second step
3. Reaction(s) taking place above 600°C: for the completion of reaction(s) in this step, considerably longer periods, even approaching 100 minutes, were needed.
Experiments conducted with the second approach revealed similar results. The weight losses obtained from the second approach were found to be nearly the same as those recorded in the first approach. However, slightly higher weight loss values were found at the end of the runs conducted with the second approach. This tendency was more pronounced in the lower temperature experiments. The weight loss values converged as the temperature increased. The weight loss difference was most prominent at the lowest experimental temperature of 250°C. At the end of 40 minutes, the continuous-to-constant-weight experiment led to a weight loss of 4.8 per cent, whereas the experiment conducted with the second approach yielded 5.3 per cent. The difference between the results is 11.5 per cent. For the same time period, the difference was 2.2 per cent for 300°C and 4.3 per cent for 350°C. At higher temperatures, the difference was found to stay in the ± 2 per cent band. The considerable difference between the weight loss values at 250°C was attributed to differences in experimental procedure as well as the reversible behaviour of the goethite-haematite transformation reaction. In the first approach, as indicated previously, the sample was removed from the furnace periodically for weighing. This did not result in a significant temperature drop, because of the short time involved. On the other hand, the interaction of the sample with the atmosphere, and therefore humidity, is longer in the first approach. Under these circumstances, it was inferred that some reverse reaction(s) takes place, and the chemically bound water that was eliminated partially integrates back into the sample, causing a lower weight loss value in the experiments conducted to constant weight. As indicated in the following paragraph, an experiment conducted to evaluate the extent of reversibility showed that the weight of the calcined sample increased by a few grams when it was exposed to the atmosphere for couple of hours. A change in the physical appearance of the ore was also observed. At all temperatures, the fawn-colored original ore became dark brown in appearance after calcination. In all runs, it was observed that the surface of the calcined sample removed from the furnace reverted to a lighter color in a short time, which could be observed easily with the naked eye. The final appearance was not the same as the original one, but the colour change is a probable indication of certain reverse reaction(s).
In order to determine which transformation(s) is (are) reversible and to quantify the degree of reversibility, an experiment was conducted as follows. A 100 g sample of dried raw ore was heated at 300°C for 2 hours and weighed. The sample was cooled to ambient temperature and was left in air for one day. It was then dried at 105°C to totally eliminate absorbed moisture. The dried sample was weighed again and the weight difference with respect to that recorded just after calcinations was determined. Similar cycles were carried out at 500°C and 800°C. The process is schematically illustrated in Figure 6.
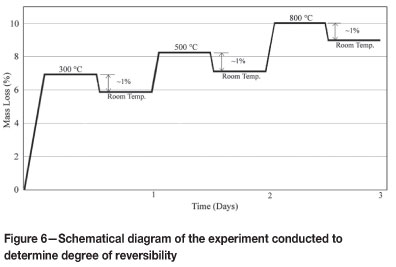
The results showed that approximately 1 per cent of the raw ore mass (i.e. approximately 10 per cent of the weight loss) was regained after cooling. This amount was constant for all temperatures. If one of the transformations occurring above 300°C was reversible, the regained mass would be greater at higher temperatures. It was therefore deduced that during thermal treatment of Sivrihisar laterites, partially reversible transformation(s) takes place below 300°C on cooling. This experiment, of course, does not yield much information about the nature and types of reaction(s) occurring below 300°C, but the regained mass can be attributed mostly to the reverse reaction of goethitehaematite transformation.
In summary, it was found that complete removal of chemically bound water and other volatiles necessitates 75 minutes at 675°C, 60 minutes at 700°C, and 45 minutes at 800°C. Under these circumstances, 700°C is recommended for effective calcination. The results of the experiments and the curves revealed that 98-99 per cent of the chemically bound water and other volatiles were removed in 40 minutes at 700°C. This degree of elimination required 25 minutes at 800°C. These periods are approximately 33 per cent and 45 per cent shorter than those required for complete elimination at 700°C and 800°C, respectively. Considering the furnace heat requirements and the power costs, 700°C and 40 minutes were determined as the optimum calcination conditions. If the calcination process was to be conducted at 800°C, the recommended period would be 25 minutes. As indicated previously, the findings of this laboratory-scale study reflect the results obtained with '-50 mm ore' and ore ground to -1 mm particle size. The effect of particle size on calcination was not investigated in the present work.
XRD analysis of calcined ore samples
Calcined ore samples were characterized by X-ray diffractometry. For this purpose, -50 mm ore samples calcined at different temperatures for a 40-minute period were stocked in small closed tubes in a desiccator. XRD analysis was carried out on samples calcined at 250°C, 300°C, 400°C, 500°C, 600°C, 700°C, and 800°C. Diffraction patterns of these samples are illustrated in Figure 7, together with a pattern from uncalcined -50 mm ore.
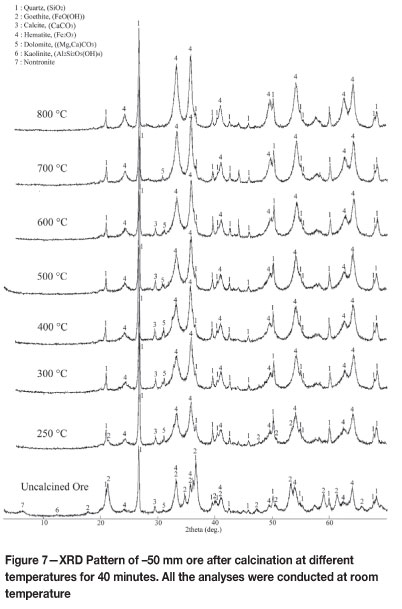
XRD analysis results showed that the uncalcined -50 mm ore consists mainly of quartz and goethite. It can be seen that a considerable amount of goethite alters to haematite at 250°C, and that this transformation is almost complete at 300°C. Although the XRD patterns did not reveal any transformation between 300°C and 500°C, TG analysis and calcination experiments recorded a mass loss in this range. This mass loss most probably corresponds to the dehydroxylation of clay minerals. However, this is not recorded by XRD analysis of calcined ore, since the percentage of clay minerals is below the detection threshold of standard XRD. Characterization of clay minerals present in the ore will be examined in a future paper.
At 600°C, a distinct peak at 29 = 43.96 (d = 2.05 A) was observed, the intensity of which decreased at higher temperatures. The nature of the reaction taking place and the phase that exists within the 600-800°C interval has not yet been identified. Comparison of XRD diagrams of calcined ores in the 600-800°C interval showed that calcite does not appear at temperatures above 700°C, because it decomposes. Normally, calcite decomposes into CaO and CO2. On the other hand, no peak related to CaO was detected. Instead, a low-intensity peak of Ca was found to appear at 28 = 28.28. Despite the preceding discussion, the nature of the reactions between 350° C and 800° C is still under examination.
Conclusions
The complete calcination process resulted in a weight loss of approximately 10 per cent of the weight of the ore. Roughly speaking, two-thirds of the weight loss is associated with the removal of chemically bound water during the goethite-haematite transformation, and the remaining one-third is related to the elimination of volatiles in the 350-700°C interval. For effective elimination of chemically bound water and all volatiles, 700°C should be selected as the calcination temperature. Forty minutes was determined as the optimum time for the particle size used in the present work. If 800°C were decided on as the calcination temperature, then a time of 25 minutes would be selected
During thermal treatment of Sivrihisar laterites, partially reversible transformation(s) takes place below 300°C on cooling
X-ray diffractometry revealed that the Sivrihisar laterites contain mainly quartz and goethite. Some haematite and calcite were also encountered in the ore. The clay minerals kaolinite and nontronite were also observed. Calcination experiments showed that 300°C was almost sufficient for the effective transformation of goethite to haematite. Calcite was reported to decompose within the 600-700°C interval. Characterization of clay minerals in the ore and phase changes between 350 and 800°C will be investigated in detail in future papers.
Acknowledgements
The authors would like to thank the Scientific and Technological Research Council of Turkey (TUBITAK) for the financial support given under Project No: 109M068, and META Nickel Cobalt Co. for supplying the lateritic ore samples from Sivrihisar.
References
1. General Mining and Metallurgical Company - LARCO. Nickel. http://www.larco.gr/nickel.php [accessed 5 Oct. 2011] [ Links ].
2. Yuksel, M. Recovery of nickel from lateritic Caldag deposit. MS thesis, Middle East Technical University, Ankara, Turkey, 1985. [ Links ]
3. Topkaya, Y.A. Nickel Extraction from Lateritic Nickel Ores Project no. 106M079. Scientific and Technological Research Council of Turkey (TUBITAK) Ankara, Turkey, 2009. [ Links ]
4. Buyukakinci, E. Extraction of nickel from lateritic ores. MS thesis, Middle East Technical University, Ankara, Turkey, 2008. [ Links ]
5. Buyukakinci, E. and Topkaya, Y.A. Extraction of nickel from Gordes lateritic ore with atmospheric leaching. ALTA 2008 Nickel/Cobalt/Conference, Perth, Western Australia, 2008. [ Links ]
6. Buyukakinci, E. and Topkaya, Y.A. Extraction of nickel from lateritic ores at atmospheric pressure with agitation leaching. Hydrometallurgy, vol. 97, 2009. pp. 33-38. [ Links ]
7. Ozdemir, V. Ferronickel Production from Manisa Caldag Lateritic Ore. Report, General Directorate of Mineral Research and Exploration, Ankara, Turkey, 2008. [ Links ]
8. Colakoglu, C., Derin, B., and Yucel, O. A study on nickel containing iron alloy production from West Anatolian region lateritic ores. EPD Congress, TMS, 2009. pp. 719-723. [ Links ]
9. Prasad, P.S.R., Prasad, K.S., Chaitanya, V.K., Babu, E.V.S.S.K., Sreedhar, B., and Murthy, S.R. In-situ FTIR study on the dehydration of natural goethite. Journal of Asian Earth Sciences, vol. 27, 2006. pp. 503-511. [ Links ]
10. Walter, D., Buxbaum, G., and Laqua, W. The mechanism of the thermal transformation from goethite to hematite. Journal of Thermal Analytical and Calorimetry, vol. 63, 2001. pp. 733-748. [ Links ]
11. Watari, F., Delavignette, P., and Amelinckx, S. Electron microscopic study of dehydration transformation. II. The formation of superstructure on the dehydration of goethite and diaspora. Journal of Solid State Chemistry, vol. 29, 1979. pp. 417-427. [ Links ]
12. Gualtieri, A.F. and Venturelli, P. In-situ study of the goethite-hematite phase transformation by real time synchrotron powder diffraction. American Mineralogist, vol. 84, 1999. pp. 895-904. [ Links ]
13. Ozdemir, O. and Dunlop, J.D. Intermediate magnetite formation during dehydration of goethite. Earth and Planetary Science Letters, vol. 177, 2000. pp. 59-67. [ Links ] ♦
Paper received Oct. 2011; revised paper received May 2012.
© The Southern African Institute of Mining and Metallurgy, 2012. ISSN2225-6253.














