Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 no.10 Johannesburg dic. 2012
PAPERS
Liquid-liquid extraction of copper (II) from chloride media by Cyanex 923 in kerosene
N.B. Devi; S. Mishra
Department of Chemistry, Institute of Technical Education and Research, Siksha' O' Anusandhan University, Odisha, India
SYNOPSIS
The extraction of Cu(II) from HCl media using Cyanex 923 in kerosene was investigated. The effect of shaking time, aqueous phase acid concentration, extractant concentration, chloride ion concentration, various metal ions (Mn2+, Mg2+, Fe2+, and Ni2+), and temperature on the extraction were studied. Using 0.1 M Cyanex 923 the extraction of Cu(II) was 6.7 per cent from 3 M HCl, which increased up to 78.5 per cent with 6.5 M HCl and then decreased due to acid extraction by the extractant. The maximum extraction (97.5 per cent) was obtained with 1 M Cyanex 923 from 5 M HCl solution. A mathematical model has been developed to describe solvent extraction equllibria over wide ranges of concentration of extractant, acid, and chloride ions. The composition of the extracted species has been predicted on the basis of slope analysis results and extraction equilibrium modelling. The negative values of enthalpy change and entropy change confirm the exothermic formation of the extracted complex.
Keywords: solvent extraction, copper, hydrochloric acid, modelling.
Introduction
Copper is an essential element and is widely used in our day-to-day life, but its presence in large concentration may be responsible for environmental pollution. For this reason its recovery from waste materials is important. Leaching of copper sulphide ores is carried out mostly in sulphate media. Consequently, large volumes of sulphate salt discharge accumulate, leading to environmental pollution. To avoid this, the chloride leaching process was developed for the treatment of Cu, Ni, Zn, and Co-containing ores. The main source of copper is chalcopyrite, which is quantitatively leached using manganese nodules as oxidant in 4 M hydrochloric acid1. Solvent extraction is a suitable technique for recovering copper from chloride solution and subsequently transferring it to sulphate solution for electrowinning. Ritcey et al.2 studied the extraction and separation of copper and zinc from chloride liquors using AGORGA P5300 extractant. Synergistic extraction using Versatic 10 and LIX 63 for separating copper from iron in high chloride concentration solution has been reported by Jhu et al.3. Organophosphorus compounds containing the phosphoryl group (P=O) have been generally used as commercial extractants for various metal ions since the 1940s. Extraction of copper(II) using acidic organophosphorous extractants has been investigated by several researchers4,5. Singh and Dhadke6 reported the extraction and separation of copper and zinc using D2EHPA and PC 88A. The extraction of Cu(II) from chloride medium using Cyanex 921 in kerosene has been reported by the authors in an earlier publication7. Cyanex 923(TRPO), a colourless liquid, is a mixture of four trialkylphosphine oxides, which exhibits extraction properties similar to that of trioctylphosphine oxide (TOPO) or Cyanex 921. As a commercial extractant, Cyanex 923 has been applied in the extraction of many metal ions from different acid media8-10. Separation of iron(III), copper(II), and zinc(II) from a mixed sulphate/chloride leach liquor using TBP, LIX 84I, and Cyanex 923 in kerosene has been studied by Sarangi et al.11. Gupta et al.12 studied the extraction behavior of 3d transition metal ions such as Ti(IV), V(IV), Cr(III), Fe(III), Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) using Cyanex 923. They also investigated the mutual separation of these metal ions using Cyanex 923 from HCl, H2SO4, and HNO3 media and reported that the extractant has good hydrolytic stability, recycling power, and loading capacity. For copper, the extracted complex was found to be CuCl2.2R. Extraction of copper from sulphate media using Cyanex 921 and Cyanex 923 has been reported by Pawar et al.13.
The present investigation deals with the study of extraction behaviour of Cu(II) ions from chloride solution by Cyanex 923 in kerosene in order to explore the possibility of recovery of Cu(II) from acidic chloride solution. The influence of equilibration time, aqueous acid concentration, extractant concentration, chloride concentration, various metal ions, and temperature on the extraction was investigated. Thermodynamic parameters such as enthalpy and entropy were calculated from the temperature variation studies. The composition of the extracted complex is proposed on the basis of slope analysis and modelling of the extraction equilibria.
Experimental
Reagents
Stock solution of copper chloride (Merck, AR grade) was prepared by dissolving the required amount in double-distilled water. One millilitre of concentrated HCl was added to the stock solution to avoid further hydrolysis. Distilled kerosene was used as organic phase diluent. The commercial extractant, Cyanex 923, TRPO obtained from Cytec Inc. of Canada, was used without further purification. All other reagents used were of analytical reagent grade.
Procedure
Equal aliquots (10 ml) of the aqueous phase containing CuCl2 solution (0.0011 M) in HCl and the organic phase with Cyanex 923 in kerosene were shaken for three minutes in a separating funnel. Complete equilibrium was achieved in one minute. The phases were allowed to settle for five minutes and were then disengaged. The copper concentration in the aqueous phase before and after the extraction was determined using an ELICO atomic absorption spectrophotometer. The distribution coefficient (D) was calculated by taking the ratio of equilibrium concentration of copper(II) in the organic phase and that in the aqueous phase. From D values, the percentage of extraction was calculated (percentage extraction = 100(D/D+1). The temperature variation experiment was carried out by mixing equal volumes of aqueous and organic phases in a flat-bottomed water-jacketed equilibration tube with the help of a magnetic stirring bar. The water in the thermostated vessel was maintained at a constant temperature (± 0.1 K) by circulating water from a constant-temperature bath through the jacket.
Results and discussion
Effect of equilibration time
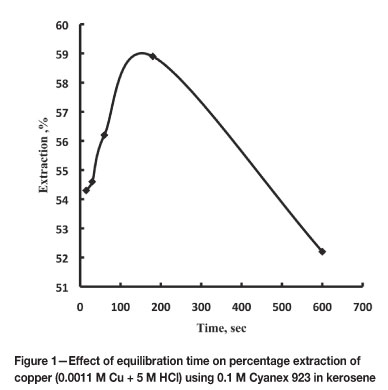
The effect of equilibration time on the extraction of Cu(II) from 5 M HCl with 0.1 M Cyanex 923 in kerosene at 1:1 phase ratio was studied. When the time was increased from 15 seconds to 10 minutes, the percentage extraction increased slowly from 54.3 per cent to 58.9 per cent and then decreased (Figure 1). The equilibration was achieved in a very short time. In all experiments, three minutes' shaking time was maintained.
Effect of acid concentration
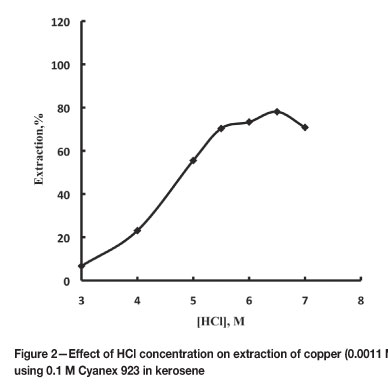
The extraction of copper(II) with 0.1 M Cyanex 923 in kerosene was studied by varying the HCl concentration from 3 M to 7 M. It has been observed that extraction depends on aqueous phase acidity. The extraction was only 6.7 per cent with 3 M HCl, whereas it increased up to 78.5 per cent with 6.5 M HCl and then decreased (Figure 2). The decrease at higher acid concentration may be attributed to the competition of HCl with metal species for extraction. At higher acid molarity, acid is extracted by Cyanex 92314.
Effect of extractant concentration
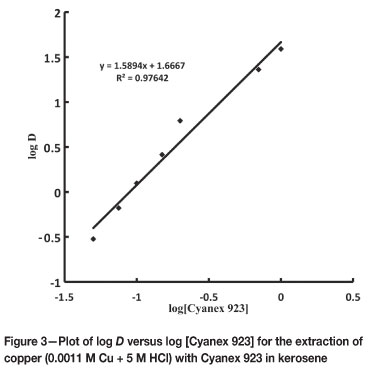
The extraction of 0.0011 M copper(II) with 0.05 M to 1.0 M Cyanex 921 in kerosene from 5 M HCl solution was carried out. The percentage extraction of copper increased with increasing extractant concentration. At 0.05 M, the extraction of copper was only 23.05 per cent, whereas it rose to 97.5 per cent at 1.0 M. The plot of log D versus log [Cyanex923] yields a slope of 1.589 (Figure 3), which reveals the incorporation of two molecules of extractant as solvating solvent in the extracted copper complex.
Effect of chloride ion concentration
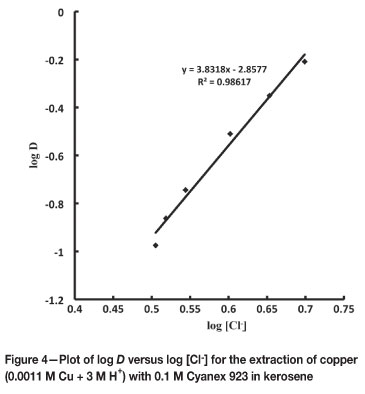
To investigate the dependence of extraction on chloride ions, the chloride concentration was varied by adding different amounts of NaCl (0.2 M to 2.0 M) with a constant concentration of H+ ion (3 M) in the aqueous phase. With 0.1 M Cyanex 923 in kerosene, the percentage extraction of copper(II) increased from 19.8 per cent to 32.3 per cent with increasing chloride ion concentration from 0.2 M to 2.0 M. This shows the association of chloride ions in the extracted copper complex. The linear plot log D versus log [Cl-] (Figure 4) with a slope of 3.83 predicts the presence of four chloride ions in the extracted species.
Effect of H+ ion concentration
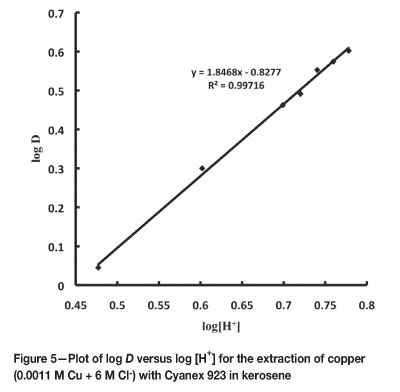
The effect of H+ ion concentration on the extraction of copper with 0.1 M Cyanex 923 in kerosene was studied by keeping the chloride ion concentration fixed at 6 M by adding calculated amount of NaCl. The extraction increased from 74.3 per cent to 80.0 per cent with a gradual increase in hydrogen ion concentration from 3 M to 6 M. Under the experimental conditions investigated, the plot of log D versus log [H+] (Figure 5) with a slope of 1.846 supports the presence of two hydrogen ions in the extracted copper complex.
Modelling of extraction equilibrium
Extraction equilibrium data has been used to develop a model for use in the metal extraction process. The correlations are based on chemical mass balance principles in which the activity coefficients of the components present in the aqueous phase and organic phase are assumed to be unity. It is assumed that all of the extractant remains in the organic phase. That is, the extractant is assumed to have no appreciable solubility in the aqueous phase. The solvating reagent can extract metal ions from chloride media in the form of neutral chlorocomplexes. In order to determine the composition of the extracted species as well as its concentration, numerical calculations were performed using the following equations, where S stands for the extractant, Cyanex 923. A relatively simple mathematical model is used to describe the solvent extraction equilibrium over wide ranges of concentration of extractant, acid, and chloride ions. The probable extraction mechanism given in Equation [1] can represent the extraction process for the system under investigation:
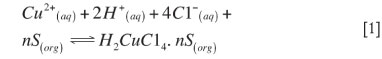
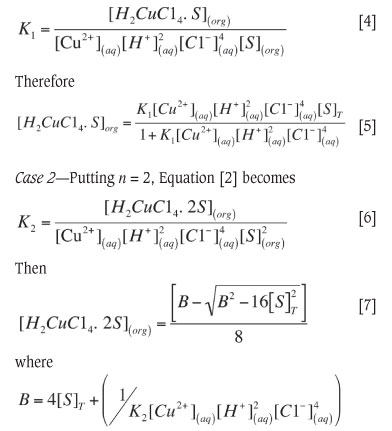
The thermodynamic equilibrium constant expression for the extraction reaction [1] was defined by Equation [2]. The mass balance Equation [3] gives the total extractant concentration in the organic phase. The equilibrium concentrations of H+ and Cl- ions used in calculation were taken as initial concentrations because of the low concentration of Cu (II).
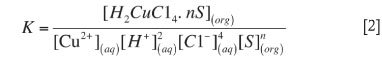
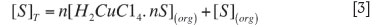
Case 1If the extraction process is described by Equation [1], then Equation [2] and Equation [3], for n = 1, will be
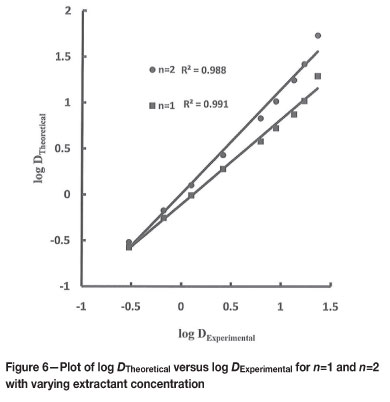
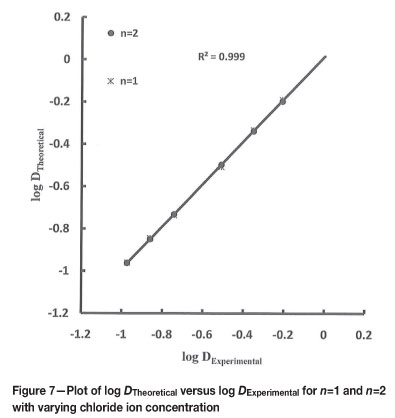
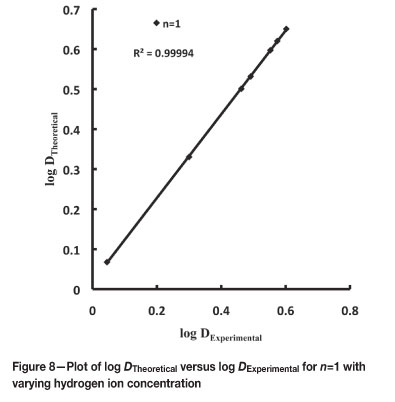
The theoretical values of distribution ratios were calculated using Equations [5] and [7] for n=1 and n=2. The plots of log DTheoretical versus log DExperimental (Figures 6 to 8) indicate that n=1 gives an acceptable fit for variation in extractant, hydrogen ion, and chloride ion concentration, and n=2 only for extractant and chloride ion concentration variation.
Effect of various metal ions
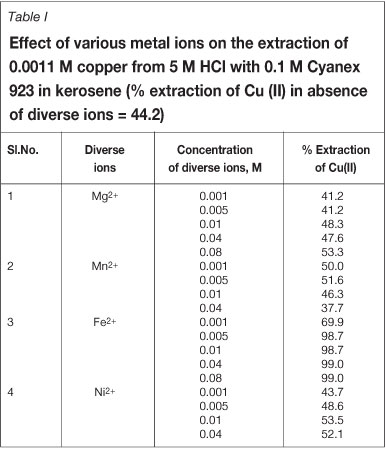
In order to assess the possible analytical applications of the recommended procedure, the effect of various metal ions on the extraction of copper(II) was studied. Copper is associated mainly with iron in chalcopyrite ore. Manganese nodules contain mostly manganese, iron, copper, and nickel. Therefore, it is quite evident that the presence of these metal ions in the aqueous phase may affect the extraction behaviour of copper.
The effect of various metal ions, Fe2+, Ni2+, Mn2+, and Mg2+, in the concentration range 0.001 to 0.08 M on the extraction of copper(II) from 5 M HCl was investigated using 0.1 M Cyanex 923 in kerosene. The extraction data for Cu(II) in the presence of individual metal ion is reported in Table I. In presence of Fe2+, the extraction of copper was increased and became quantitative with 0.005 M iron ions. In the presence of Mn2+ the extraction of Cu(II) decreased from 50 per cent to 37 per cent. All other metal ions have negligible interference with copper extraction. Hence, Cyanex 923 can be beneficial in extracting copper(II) from HCl medium even in the presence of Mg2+, Fe2+, and Ni2+ ions.
Effect of temperature
The effect of varying temperature on the extraction of Cu2+ with Cyanex 923 was studied at fixed concentrations of HCl (5 M) and Cyanex 923 (0.1 M) in kerosene. The distribution ratio of Cu2+ ion decreased with increase in the experimental temperature, from 55.5 per cent at 300 K to 38.6 per cent at 333 K. The change of the extraction equilibrium constant (Keq) with temperature is expressed by:

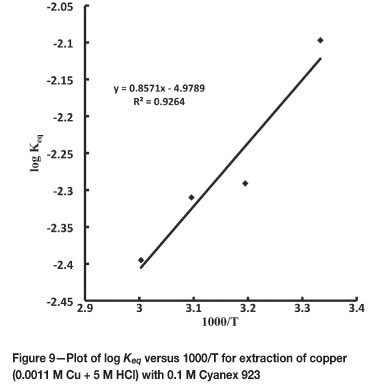
The plot of log Keq versus 1000/r (Figure 9) gives a straight line with slope of -ΔH/2.303R and an intercept of ΔS/2.303R. For Cu (II) under the given extraction conditions, ΔH was found to be -16.4 kJ mol-1 and ΔS to be -95.314 J K-1 mol-1. This indicates that the process is exothermic, accompanying a decrease in the randomness of the extraction system.
Conclusions
1. From the above investigation it is concluded that the extraction of Cu(II) increases with an increase in HCl concentration up to 6.5 M and thereafter decreases.
2. The extraction percentage increased with an increase in Cyanex 923 concentration, being 94.4 per cent with 0.4 M Cyanex and 97.5 per cent with 1 M Cyanex from 5 M HCl. From our earlier work it was found that 0.5 M Cyanex 921 extracts 93 per cent Cu(II) from 5 M HCl. Therefore, it is concluded that the extraction efficiency of Cyanex 923 for Cu(II) is higher than that of Cyanex 921 in chloride media.
3. The temperature dependence studies of the distribution ratio gave rise to negative values of ΔH and ΔS, indicating release of heat during complexation.
4. The extraction data have been analysed both graphically and numerically to determine the stoichiometry of extracted species and the distribution ratios. The slope analysis results revealed the formation of the disolvated species, H2CuCl4.2Cyanex 923. The modelling of extraction data was in agreement with the slope analysis results for extractant and chloride concentration variation, whereas deviation was observed in case of H+ concentration variation.
5. The extraction of copper increased in the presence of Fe2+, whereas it decreased in the presence of Mn2+. Ni2+ and Mg2+ ions did not have any significant adverse effect on the extraction.
Acknowledgements
The authors are thankful to Cytec Inc., Canada, for providing the gift sample of Cyanex 923. The authors are also grateful to authorities of Siksha'O'Anusandhan University for the encouragement to carry out this research work.
References
1. Devi, N.B., Madhuchhanda, M., Rath, P.C., Rao, K.S., and Paramguru, R.K. Simultaneous leaching of deep-sea manganese nodule and chalcopyrite in hydrochloric acid. Metallurgical and Materials Transactions B, vol 32B, 2001. pp. 777-784. [ Links ]
2. Ritcey, G.M., Lucas, B.H., and Price, K.T. Evaluation and selection of extractants for the separation of copper and zinc from chloride leach liquor. Hydrometallurgy, vol. 8, no. 3, 1982. pp. 197-222. [ Links ]
3. Zhu, Z., Zhang, W., and Cheng, C.Y. A synergistic solvent extraction system for separating copper from iron in high chloride concentration solutions. Hydrometallurgy, vol. 113-114, 2012. pp. 155-159. [ Links ]
4. El-Hefny, N.E. and Daoud, J.A. Extraction of copper(II) by Cyanex 302 in kerosene from different aqueous media. Solvent Extraction and Ion Exchange, vol. 25, 2007. pp. 831-843. [ Links ]
5. Sole, K.C. and Hiskey, G.B. Solvent extraction of copper by Cyanex 272, Cyanex 302 and Cyanex 301. Hydrometallurgy, vol. 37, 1995. pp. 129-147. [ Links ]
6. Singh, R.K. and Dhadke, P.M. Extraction and separation of zinc(II) and copper(II) from Perchlorate media. Journal of the Serbian Chemical Society, vol. 67, no. 1, 2002. pp. 41-51. [ Links ]
7. Mishra, S. and Devi, N.B. Extraction of copper(II) from hydrochloric acid solution by Cyanex 921. Hydrometallurgy, vol. 107, 2011. pp. 29-33. [ Links ]
8. El-Ammouni, E. and Instin, P.A. Hafnium extraction from acidic chloride solutions by Cyanex 923. Solvent Extraction and Ion Exchange, vol. 14, no. 5, 1996. pp. 871-887. [ Links ]
9. Ansari, S.a., Murali, M.S., Pathak, P.N., and Manchanda, V.K. Separation of iron from cobalt in nitrate medium using Cyanex 923 as extractant. Journal of Radioanalytical and Nuclear Chemistry, vol. 262, no. 1-2, 2004. pp. 469-472. [ Links ]
10. Reddy, M.L.P., Varma, R.L., and Ramamohan, T.R. Cyanex 923 as an extractant for trivalent lanthanides and yttrium. Solvent Extraction and Ion Exchange, vol. 16, no. 3, 1998. pp. 795-812. [ Links ]
11. Sarangi, K., Parhi, P.K., Padhan, E., Palai, A.K., Nathsarma, K.C. and Park, K.H. Separation of iron(III), copper(II) and zinc(II) from a mixed sulphate/chloride solution using TBP, LIX 841 and Cyanex 923. Separation and Purification Technology, vol. 55, 2007. pp. 44-49. [ Links ]
12. Gupta, B., Deep, A., Malik, P., and Tandon, S.N. Extraction and separation of some 3d transition metal ions using Cyanex 923. Solvent Extraction and Ion Exchange, vol. 20, no. 1, 2002. pp. 81-96. [ Links ]
13. Pawar, S.d., Mishra, B.Y., and Dhadke, P.m. Solvent extraction of copper(ii) from sulphate media using neutral extractants Cyanex 921 and Cyanex 923. Journal of the Indian Chemical Society, vol. 78, no. 8, 2002. pp. 681-683. [ Links ]
14. Alguacil, F.J. and Lopez, F.A. The extraction of mineral acids by the phosphine oxide Cyanex 923. Hydrometallurgy, vol. 42, 1996. pp.245-255. [ Links ] ♦
Paper received Jan. 2012; revised paper received May 2012.
© The Southern African Institute of Mining and Metallurgy, 2012. ISSN2225-6253.














