Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 no.7 Johannesburg jul. 2012
JOURNAL PAPER
Operation of a concentrated mode dual-alkali scrubber plant at the Lonmin smelter
G.A. BezuidenhoutI; J. DavisI; B. van BeekI; and J.J. EksteenII
ILonmin (Western Platinum Ltd), Marikana, South Africa
IIDepartment of Metallurgical Engineering, Western Australia School of Mines, Curtin University, Perth Australia
SYNOPSIS
Lonmin Platinum installed a concentrated mode dual-alkali scrubber at the smelter in Marikana in 2002. The dual-alkali scrubber was the technology of choice at that time as a sulphur fixation plant, due to the perceived benefits of handling both the high SO2 concentrations of converter off-gas and the swings in SO2 concentration that are linked to Peirce-Smith operation. Owing to current and impending legislative requirements for air quality and waste, Lonmin is currently considering additions to the dual-alkali plant, but is also evaluating alternative technologies for sulphur fixation. This paper reviews the decision of Lonmin to install a concentrated mode dual-alkali scrubber and presents plant performance achieved. It also describes the important control variables and sensitivities of the plant, and the final product that is produced by the operation of the plant. The legislative requirement that drives the Lonmin technology evaluation is also discussed.
Keywords: Lonmin smelter, concentrated mode dual-alkali operation, sulphur abatement.
Background and design
Lonmin is an integrated mine-to-market primary producer of platinum group metals, and its smelting operations are based close to Marikana in the North West Province of South Africa. Merensky and UG2-type ores are concentrated through a flotation process and then smelted in a three-electrode AC furnace operation. Matte-forming phases separate from gangue material to form a distinct furnace matte and slag. During the smelting operation labile sulphur is given off in the furnace off-gas. The amount of labile sulphur is driven largely by the ore type and the amount of pyrite present in the ore. The reactions that produce labile sulphur and the corresponding temperatures where the sulphide minerals partially decompose are discussed by Eksteen (2010). Typical SO2 concentration in furnace off-gas is about 0.9 per cent (by volume on a dry basis), but this concentration varies per furnace and depends on dilution (ingress air) and can be as low as 0.3 per cent. A slight negative pressure is maintained in the furnace freeboard to collect dust and gas species from the smelting operation. Eksteen et al. (2011) reviewed the Lonmin smelter operations and how flue gas formation and choice of flue gas desulphurization technology is related to Lonmin's history of UG2 processing.
Furnace matte is tapped into ladles and transported by an overhead crane to Peirce-Smith converters. In the converting operation, air is injected into the liquid bath in order to oxidize FeS and remove Fe from the converter matte, which is refined in the base metal removal plant. During the converting process, SO2 concentration from the liquid bath is very constant and in the order of around 13.5 per cent according to FactSage modelling by Bezuidenhout et al. (2010). Lonmin typically achieves a dilution air ratio in the converter gas of between 3:1 and 2:1 (three units of air drawn in through the hood for one unit of air injected in the converter bath). Actual converter off-gas measurements confirm that SO2 concentrations can vary from 3.0 per cent to 6.0 per cent in converter off-gas due to the dilution effect. Typical operation at Lonmin will see a converter in stack about 12 hours in a 24-hour period.
Since the Peirce-Smith converter operation is a batch-type process, overall smelter SO2 concentrations vary considerably with time, with zero, one or two converters that can be blowing at any time. Fixating sulphur from an off-gas stream that varies widely in both volume and SO2 concentration is a challenge for any technology that is currently available.
Lonmin requirements and business philosophy in 2002
Lonmin installed the first SO2 abatement equipment in 2002. Hatch was contracted to perform a technology evaluation and was subsequently appointed as the EPCM contractor during erection of the plant. At the time Hatch recommended a concentrated mode dual-alkali plant, as the technology was firmly established (since the mid-1970s) and at the time there were more than 50 installations globally. The concentrated mode dual-alkali plant was considered the only technology that could achieve good scrubbing efficiency with SO2 concentrations of up to 3 per cent (with installations handling SO2 concentrations as high as 9 per cent), and which could handle low SO2 concentrations when no converter was in stack. The dual-alkali technology was developed in order to overcome the disadvantages inherent to lime and limestone scrubbing (such as scaling and low reactivity), while retaining the perceived advantage of producing a product that could be sold or disposed of. Another major advantage of the dual-alkali technology is the low energy requirements (low solution flow rates required and no heating/cooling requirements).
Acid production was not considered viable due to the minimum SO2 concentration input to the plant to maintain autothermal oxidation. Ca-based scrubbing was not considered viable due to the high input SO2 concentration and the required SO2 removal efficiency of at least 96 per cent. Technologies where SO2 was absorbed in an intermittent medium (like organic amines) before it was released in a controlled manner (and thereby smoothing the swings of a batch operation) were only just emerging.
The product from the dual-alkali plant is a mixture of calcium sulphite and calcium sulphate. As there was a reference to this product being disposed to tailing dams (for instance, at Barrick Goldstrike in Nevada, USA), Lonmin planned to co-dispose the product of the dual-alkali plant together with tailings from the concentrator operations to active tailings dams.
A variable throat scrubber was installed together with the dual-alkali plant in order to remove particulates and to quench the gas before the dual-alkali plant.
Design summary
The off-gas cleaning plant was designed with the overall objectives given in Table I.
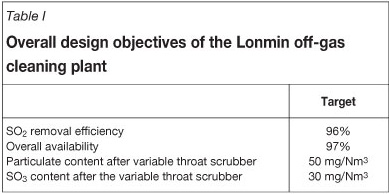
The total design off-gas volume was 173 000 Nm3, with SO2 concentrations up to 3 per cent. The dual-alkali plant was designed to remove up to 100 tons of SO2 per day.
Lonmin equipment description
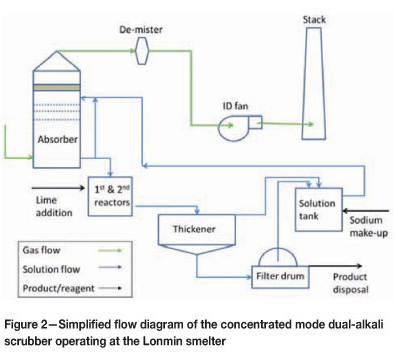
Figure 1 shows a block flow diagram of the off-gas handling equipment installed at Lonmin. Figure 2 shows a simplified flow diagram of the dual-alkali system.
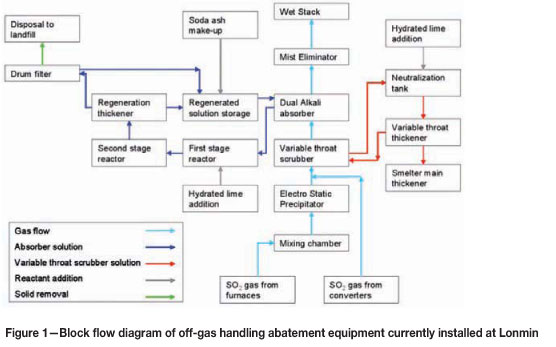
Off-gases from all the furnaces are combined in a mixing chamber before passing through a two-field electrostatic precipitator (ESP ). Typical removal efficiency of particulate matter in the ESP is around 99 per cent. Off-gases from the converter are combined with the furnace off-gas after the ESP but before the variable throat scrubber. During the initial design the converter gases were also passed through the ESP, but the high gas flows from the converter lowered overall particulate removal efficiency across the ESP. A testing campaign on converter off-gases confirmed that the particulate sizes in converter off-gas is coarser and has a much lower particulate loading, compared to furnace off-gas. A decision was taken to allow converter gas to by-pass the ESP and improve the efficiency of furnace gas removal.
The variable throat scrubber quenches the gas and removes particulate matter. Pressure drop across the variable throat scrubber is designed between 4 kPa and 8.5 kPa. Weak acid produced in the variable throat scrubber is neutralized with hydrated lime and sent to a thickener. Underflow from the thickener is returned to the blending section (as it contains valuable particulates), while the overflow is returned to the variable throat scrubber. Chevrons are present in the scrubber to limit water droplet carry-over to the absorber circuit.
The quenched gas then passes through an absorber. The absorber design is based on a countercurrent flow of gas and solution. There are four perforated horizontal trays in the absorber and the typical pressure drop across the absorber is 2 kPa to 3.5 kPa. After the absorber, the gas passes through a mist eliminator and then out the wet gas stack. Two continuous SO2 monitors measure SO2 concentrations on the stack, together with gas flow rates and temperature. Two fans are run in parallel and overall draught control in the system is done by maintaining set pressures directly after the ESP. Pressure set-points vary according to the number of converters in stack.
Fresh solution is pumped to the absorber based on pH measurement (done in a pH pot on the recycle line). The design pH control in the absorber is between 6.0 and 6.5. Typical feed rates of fresh feed solution to the absorber are between 350 m3/h to 550 m3/h with converters in stack, but can drop to 60 m3/h with very low SO2 concentrations in the gas. The recycle pumps continuously recycle solution in the absorber, but bleed pregnant solution from the absorber based on a level control loop in the absorber.
The pregnant solution is fed into a baffled and well-stirred first-stage reactor where lime is added as a powder hydrated lime by means of a screw feeder. Lime addition is also controlled through a pH measurement control loop, but the pH meters are situated in the second-stage reactor. pH control on the reactors is set at between 11.2 and 11.8. The first-stage reactor has a volume of around 31 m3 and a retention time of around 4 minutes at peak flow rates.
The first-stage reactor overflows into a larger second-stage reactor that is also baffled and well stirred. The effective volume of the tank is around 180 m3, and the retention time is around 20 minutes at peak flow rates. The second-stage reactor overflows into the thickener feedwell with an open launder. The thickener can take around 2000 m3 of slurry and has a diameter of 25 m. Thickener overflow is stored in a stirred regenerated solution tank that can hold around 500 m3 of solution.
Underflow is pumped from the thickener to a filter feed tank. Slurry SG and flow rate is measured on the underflow line. Lonmin is currently operating two vacuum drum filters to remove moisture from the filter cake. No washing is done on the filter cake, and filtrate are returned to the regenerated solution tank. Filter cake from the filters is stored in a storage shed and loaded with front end loaders into tipper trucks that transport the filter cake to a hazardous landfill site.
Sodium make-up to the system is done by means of soda ash that is mixed with regenerated solution. Make-up is done on a batch basis once a day. The amount of soda ash added is decided on based on the SG of the regenerated solution.
Lonmin is currently installing secondary hoods on the converters in order to capture fugitive gases from the converter operation. Fugitive gases will be captured, measured, and stacked from the existing concrete stack only for the moment. The design is based on a horizontal telescopic hood movement system that installed at more than five plants worldwide.
Concentrated mode dual-alkali plant basics
In this section, the basics of the concentrated mode dual-alkali plant will be discussed.
Chemistry overview
A dual-alkali plant gets its name from using two alkali species to achieve different stages of the process: sodium (Na) species are responsible for the absorption of SO2, while calcium (Ca) species precipitate in order to regenerate Na species and bind the sulphur in a mixed calcium sulphite (CaSO3) and calcium sulphate (CaSO4) crystal.
Two kinds of absorbers are commonly used in the dual-alkali system: the venturi scrubber and the tray tower absorber. The venturi type is desirable where particulate control is necessary because it can be used alone for both SO2 and particulate removal. If SO2 removal greater than 95 per cent is required, a tray tower absorber can be used (very often in combination with a venturi). The overall absorption of SO2 is done by sodium sulphite forming sodium bisulphite according Equation [1]:

The absorptive capacity is increased by the presence of some caustic in the system, formed during precipitation according to the equilibrium reaction (Equation [6]). The extent of caustic formation is promoted by high pH (above pH 12) and by lower concentrations of Na2SO3.
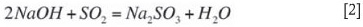
According the Arthur Little EPA report (1977), elevated temperatures during absorption tends to decrease SO2 absorption efficiency due to elevated SO2 partial pressures. The higher temperatures also tend to increase oxidation rates according to Equation [4].
Sodium is made up to the system (due to losses in the moisture associated with the filter cake) in the form of soda ash and participates in the absorption reaction according to Equation [3]:
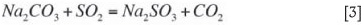
The sum total of the sodium species in Equations [1-3] is sometimes referred to total oxidizeable sulphur (TOS) and represents active sodium concentrations in solution. Although sodium bisulphite cannot directly absorb SO2, it is considered as an active species (and included in the TOS value) as it can be regenerated by the addition of lime (or finely powdered limestone).
Some oxidation of Na2SO3 takes place in the system and forms Na2SO4 (which is inert for any further SO2 absorption) according to Equation [4]. According the Arthur Little EPA report (1977), the rate of oxidation or oxygen transfer in the absorber is promoted by a higher oxygen concentration in the flue gas and a higher flue gas temperature. The nature and concentration of species in the scrubbing solution strongly influence oxidation rates (with higher TOS levels suppressing the rate of oxidation). The amount of sulphate forming is absolutely crucial for system performance and will be explored in more detail in the section on operational experience.
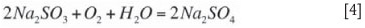
Pregnant solution from the absorber tower is pumped to the precipitation section, which consists of a stirred first-stage reactor (where Ca additions are made), overflowing to a stirred second stage-reactor (providing residence time for crystal growth), overflowing to a thickener for solid-liquid separation. Ca additions are done based on pH control with the pH probes installed in the second-stage reactor.
Precipitation can be divided into a number of reactions that occur simultaneously. The first and most important precipitation reaction takes place during neutralization of the sodium bisulphite according to Equation [5] and goes to completion. Dreamel (1973) found the driving force for this reaction is improved by the pH difference to neutralization and is suppressed by high concentrations of sulphates in solution. Dauerman and Rao (1979) showed that the reaction in Equation [5] occurs almost instantaneously, but can be influenced by mixing. The neutralization reaction forms massive precipitation in a very short period and is supposed to form large aggregates that can settle quickly in the thickener. Equation [5] will proceed until neutrality is reached, which is at a pH of about 8.5. At this point, most of the NaHSO3 has been converted to Na2SO3. This reaction forms the basis of the smaller first-stage reactor that is well mixed.
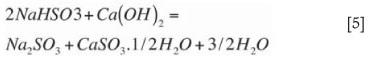
Sodium sulphite further participates in precipitation according to the equilibrium reaction (Equation [6]. As this is an equilibrium reaction, it tends to have a lower driving force than Equation [5], forming smaller crystals at a slower rate that do not grow easily. Settling of this later-forming calcium sulphite is more difficult. This reaction is promoted by lower Na2SO3 levels and higher pH levels. Tseng and Rochelle (1986) reported that calcium sulphite hemihydrate crystal growth rate is suppressed by lower temperatures and by higher sulphate (SO42-) concentrations. Equation [6] forms the basis of the larger (longer residence time) stirred second-stage reactor. The equilibrium reaction should not continue into the thickener, as this will form small crystals that do not settle well. Low lime reactivity will allow the reaction to run in the thickener, and high hydraulic flow rates will shorten the residence time in the reactors. pH control in the reactors above 12 will also allow the equilibrium reaction (Equation [6]) to continue into the thickener. NaOH should be only a minor fraction of the absorptive capacity. The intent of operating the reactors at a pH in the range of 11.2 to 11.8 is to provide overall stability to pH control in the regeneration circuit. Attempts to operate at any pH between 8.5 and 11.2 result in wide swings in reactor pH because the chemistry is completely unbufferred.
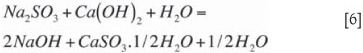
The sodium sulphate formed by oxidation of sodium sulphite Equation [4] also precipitates according to Equation [7]. Under normal operating conditions, the ratio of calcium sulphate precipitation to calcium sulphite precipitation will reflect the ratio of sodium sulphate to sodium sulphite present in the liquor solution system. This ratio is referred to as the total system oxidation and typical concentrated mode dual-alkali operation can handle system oxidation up to 25 per cent. The sulphite and sulphate concentrations (system oxidation) also have a profound effect on the physical properties of the calcium/sulphur solids settling and filtration characteristics. Most of the co-precipitation of calcium sulphate in the calcium sulphite crystal lattice occurs in the neutralization precipitation reaction (Equation [5]) because of the high rate of the reaction and the presence of SO42- ions occluded in the calcium sulphite crystal matrix.
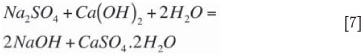
LaMantia et al. (1976) reported that most of the oxidation occurs in the scrubbing tower (absorber). They estimate that up to 90 per cent of oxidation takes place in the absorber. Recycle flow in the absorber should be kept to a minimum (still allowing effective SO2 absorption) in periods of high O2/SO2 ratios.
Product of plant
The product from the dual-alkali plant is mostly a combined crystal of CaSO3.½H2O and CaSO4.2H2O that form according to Equations [5-7]. Since this product contains a mixture of calcium sulphite and sulphate, it is often referred to as CaSOx. As already mentioned, the ratio of SO32- to SO42- in the product reflects the ratio in the liquor, and therefore the oxidation state of the system (up until about 25 per cent, where the removal of SO42- cannot keep up with SO32- ). At this point the system becomes unstable as the sulphate will suppress sulphite precipitation and the particle size and amount of solids produced will not be conducive to settling and filtering.
The solid particle density of calcium sulphite is around 2.4 g/cm3, while that of calcium sulphate is around 2.3 g/cm3.
Calcium sulphite crystals are highly porous and spongelike calcium sulphite crystals can retain a great deal of water. These rather fragile crystals break under pressure and release the water. Thus, calcium sulphite sludge is thixotropic and tends to become fluid with vibration or stress.
The CaSOx filter cake from the dual-alkali plant contains moisture and soluble salts (notably sodium salts). According to the Arthur Little EPA report (1977), insoluble solid content of filter cake ranges from 45 per cent to 75 per cent. They report that 0.5 wt% to 1 wt% of soluble sodium salts are occluded in the calcium crystals and cannot be removed, disregarding the number of washing cycles used during filtration.
The long-term average moisture content of Lonmin filter cake is 41.9 per cent with a standard deviation of 10.3. The sodium content of the filter cake is 2.63 wt% (wet basis), with a standard deviation of 1 .36.
Reagent consumption
Sodium is introduced to the dual-alkali plant in the form of soda ash (sodium carbonate or Na2CO3). Sodium make-up is necessary due to sodium losses from the system. The losses are largely driven by moisture carry-over with the CaSOx product after filtration.
Soda ash is received in fine powder form by road tanker and pneumatically transferred to a storage silo. Soda ash make-up is currently done on a batch basis once a day by first dissolving the soda ash in a tank filled with regenerated solution and then bleeding the make-up solution back to the main absorber solution storage tank. Mole sodium consumption is around 0.18 mole Na per mole S removed as a long-term average, with a standard deviation of 0.09.
According the EPA Summary report (1980) Na mole usage per mole S removed is around 0.05 for most systems designed in the USA, while Japanese systems achieve figures as low as 0.02. It is mentioned that the Na mole usage per mole S removed can increase to 0.15 if the filter cake is not washed. In the case of Lonmin, no washing is done on the filter cake.
The water balance in a properly designed dual-alkali system will allow enough water for three to five cycles of washing during filtering, as water is lost from the closed loop system by flue gas saturation, by occlusion with the solid waste, and by water crystallizing in the solid waste. In the case of Lonmin, most of the saturation of the flue gas takes place in the variable throat scrubber circuit. The water loop in the variable throat scrubber contains valuable solid particles that need to be recovered and the water loop is therefore kept separate from the dual-alkali water loop. Water from the dual-alkali circuit is lost mostly with the solid product. The use of washing water would therefore be limited to between one and three washing cycles, although Lonmin has not done washing for a number of years. The reason for this is both the antiquity of the filter drum equipment, as well as the frequent problems experienced with poor crystal quality and the resulting thin, wet cakes produced by the filter drum.
Lime requirements for sulphur fixation are supplied by slaked/hydrated lime (Ca(OH)2) addition. The slaked lime is received as a fine powder in road tankers and pneumatically transferred to a storage silo. Calcium is added by a screw feeder directly into the first-stage reactor and the addition is controlled by a pH meter situated in the second-stage reactor. Calcium consumption is around 1.105 mole per mole S removed as a long-term average, with a standard deviation of 0.114. The EPA Summary report (1980) gives typical calcium usage of 1 mole per mole sulphur removed for a number of systems installed in the USA.
Mole calcium usage per mole sulphur removed is also referred to as lime utilization. Dauerman and Rao (1979) found that strong initial mixing of lime with the sodium bisulphate solution can improve lime utilization. Lime addition starts with a solid reactant (powder hydrated lime) and ends with a less soluble product, calcium sulphite. If the lime addition is not properly mixed with the solution, the reaction in Equation [5] can form aggregates around the hydrated lime reactant and thereby lower the lime utilization. According to the Arthur Little EPA report (1977), lime utilization is also negatively influenced by higher sulphate concentration (system oxidation), higher pH control in the reactors, and by poor lime reactivity.
In a concentrated mode dual-alkali scrubber, calcium addition to the system can be done by either slaked lime or limestone. According to Dauerman and Rao (1979), limestone is less reactive than lime during regeneration by about an order of magnitude, and would therefore require longer residence time in the regeneration circuit (with around double the reactor and thickener capacity requirement as compared to use of lime) . Impurities in limestone, especially magnesium, seriously impair the settling properties of solids. Calcium utilization rates are also lower and the proportion of sulphate in mixed-crystal solids is smaller than in systems regenerated with lime. Because limestone is less soluble than calcium sulphite, OH- will not be regenerated from SO3-2 according to Equation [6]. Limestone that is amorphous in nature, rather than crystalline, is reported to be more reactive during dual-alkali regeneration. Limestone is, however, considerably less expensive than lime, and might present an opportunity for a lower cost system during design. However, retrofitting an existing system running on slaked lime to be able to use limestone might not be possible, due to footprint and plant integration limitations.
Actual performance achieved at Lonmin smelter
In this section, actual achieved availabilities and removal efficiencies will be compared to the design of the dual-alkali plant and reasons for the deviations will be discussed.
Availabilities
Currently Lonmin does a bi-annual planned shutdown for maintenance and cleaning of the off-gas system. The total time lost during the bi-annual shutdown is around 10 days, and would represent a non-availability of 2.7 per cent for planned shutdowns. Availability of the absorber circuit itself can easily achieve 100 per cent in months where no planned shutdowns are done. The long-term plant availability for Lonmin is 96.4 per cent. The main reason for unplanned shutdowns is related to the following two items:
- Scaling in the variable throat scrubber circuit. If the variable throat scrubber needs to be taken down to clear scaling, the absorber circuit is taken down as well
- Periods of crystal carry-over in the thickener overflow and resulting scaling in the absorber circuit.
Removal efficiencies
Long-term SO2 removal efficiency achieved by the Lonmin dual-alkali plant is 85 per cent. The reason why availabilities basically achieve design target, but removal efficiency does not, is related to the fact that the dual-alkali plant is mechanically available, but other factors force the plant to be operated on recycle. The other factors are listed below. In this context, recycle means that fresh feed of solution is cut off from the absorber tower and the pH of the recycled solution is allowed to drop to about 2. With such a low pH, removal efficiency is almost negligible and no sulphur fixing is achieved. The system is normally operated in recycle state until the solids removal circuit stabilizes and new solids can be formed. Fresh feed is added to the absorber the moment the cause has been rectified. The major causes leading to poor removal efficiency are:
- Lonmin is currently using road transport for removal of filter cake. Availability of road transport side tippers has led to a number of periods where filter cake could not be removed from site. Lonmin has built a large shed for storage of filter cake in order to act as a buffer between the drum filter and the loading of trucks
- Lime supply has been interrupted a number of times during the life of the plant. A lime storage silo that can hold around 10 days' supply of lime was built with the dual-alkali plant
- Settling and filtering problems associated with type and size of crystals produced have occurred frequently. Settling and filtering problems do not allow solids to be removed from the system, and force the system into recycle until the enough solids is removed to continue with absorbing. Lonmin installed a second drum filter to assist with solids removal and is currently investigating flocculant addition to improve settling. The following section contains a full discussion on the operational experience and initiatives at Lonmin..
Operational experience
This section discusses some of the focus areas on the operation of the dual-alkali plant.
Neutralization in variable throat scrubber circuit
The variable throat scrubber is operated on a separate water circuit from the dual-alkali plant, due to the value of the solids captured and the necessity to blend the solids back to the smelting circuit. Both SO3 and SO2 will dissolve into water to form a weak acid. This weak acid stream is neutralized with hydrated lime, which precipitates as gypsum when the solubility limit is exceeded.
During the design phase of the variable throat scrubber, the build-up of Ca2+ and SO42- ions in the water circuit was not properly catered for, with the result that gypsum solubility limits are frequently exceeded. Gypsum precipitation in the scrubber, pipes, and pumps forces frequent stops to open lines and de-scale with high-pressure water. Recirculation and bleed pumps often receive mechanical damage due to pieces of scale that damage the impeller.
The poor performance of the variable throat scrubber directly influences the dual-alkali plant through:
Necessitating a bi-annual shut to clean scaling
Not properly removing particulates and SO3 in times of low flow or incorrect pressure drop.
Lonmin is currently investigating the option to stop neutralizing the weak acid with hydrated lime, but to rather pump the variable throat thickener overflow (devoid of precious solids) to a tailing line. PGM concentrator tailings have a large buffer capacity, and it is common industry practice to neutralize weak acid with tails. The addition of a flocculent will be necessary in the variable throat thickener to ensure that no solids are lost to the tailings.
Chemistry control on the dual-alkali plant
Good chemistry control on the plant allows the precipitation of the correct crystal morphology to ensure good settling and filterability. It is the experience of Lonmin that stable control of the dual-alkali plant can be achieved if the properties of the crystals produced can be controlled. The dual-alkali plant is very sensitive to sudden changes in chemistry and needs to be operated with tight control.
The pH in the absorber is determined primarily by the ratio of sodium sulphite to sodium bisulphate. When the system is operating properly, the ratio of sulphite to bisulphite will be maintained between bisulphite formation (as shown in Equation [1]) and fresh solution feed (containing sodium sulphite and hydroxide). The system will be sufficiently buffered to maintain the pH at a setpoint of between 6 and 6.5.
As the pH is allowed to fall below about 6, SO2 capture efficiency reduces dramatically. If the pH is allowed to run higher than 6.5 (but still below 8.5), the neutralization reaction (Equation [5]) will have a low driving force and a subsequent slow rate of reaction. Calcium salts precipitated will be primarily from the equilibrium reaction (Equation [6]) and the crystals agglomerates formed will be smaller. Above a pH of about 9, scaling in the absorber will result from carbon dioxide absorbed from the flue gas precipitating as CaCO3 scale. Equation [6] will also tend to form calcium sulphite crystals, especially if the TOS values are low.
The dual-alkali plant at Lonmin was designed to operate at a TOS level of between 20 g/L and 25 g/L. TOS measurement can be done by acid/base titration. Lonmin stopped TOS measurements a number of years ago and is currently doing only SG measurements of the solution. With a conversion factor, this provides an indication of the total sodium in solution. Sodium addition at Lonmin is based only on total sodium measurements. This strategy works well enough when the plant throughput is stable and the system is in equilibrium and running well.
However, when system oxidation changes, so does the active (TOS) to inactive sodium ratio. The effect is that the smelter is not able to control buffering in the pH by maintaining TOS levels. If the solution has a low TOS (not properly buffered), the pH will decrease very quickly (due to the formation of acidic ,bisulphite) and the fresh feed flow through the absorber will be increased in an effort to control the pH. The effect of this is very high system flows, which reduces residence time in the reactors and carries precipitation over into the thickener.
Sodium losses occur constantly as moisture is lost with the filter cake during 24-hour operation. Lonmin is currently doing batch make-up of sodium (by adding soda ash) once per day. The effect is that sodium levels will tend to vary during a 24-hour period. Apart from pH buffering in the absorber, the higher TOS concentrations will serve to suppress oxidation by Equation [4] and influence precipitation behaviour by suppressing gypsum precipitation and caustic formation as per Equations [6] and [7]. Both of these reactions, coupled with a high flow rate, tend to produce the finer crystals that form in the thickener.
High TOS buffering is only part of the picture. The other part would be the SO2 concentration and the total gas volume flow, which is highly variable. The Lonmin operation can have zero, one, or two converters in stack at any given moment. Total SO2 input to the absorber can therefore vary with a factor of 8 when two converters turn into stack (from a baseline of zero converters in stack). In an effort to prevent the pH from decreasing, Lonmin implemented a feed-forward control system that can react on converter rotation and proactively increase system flows to stabilize the pH.
When the dual-alkali plant was built, it was decided to install butterfly valves in the absorber feed and bleed lines, which are known to have poor flow control ability. The butterfly valves were replaced with pinch valves in 2009, resulting in an improvement of flow control. A minimum opening position in the fresh feed line was also found to allow the pH to become too high in periods of low SO2 concentration in the gas stream, and was subsequently changed.
pH control on the regeneration circuit is less variable than on the absorbing circuit. Lonmin is currently feeding powdered slaked (hydrated) lime through an open screw feeder arrangement directly into the first-stage reactor. Large upsets in pH are caused only when feeding is stopped due to a blockage in the screw feeder (or another part in the feeding circuit), but this happens rarely. In periods where flow rates of solution are high through the reactors (due to low high SO2 input and low TOS values) lime addition is quite rapid. As described by Dauerman and Rao (1979), solid calcium might not have sufficient time to mix and dissolve, but can be occluded in the calcium sulphite crystal. Overfeed of lime will also tend to increase the pH, which will advance the equilibrium reaction (Equation [6]) and will increase Ca2+ concentrations that will allow gypsum precipitation. Both of these situations will have an adverse influence on crystal morphology. For this reason, in periods where crystals are small and do not settle well, it is best to decrease pH in the regeneration circuit to around 11.2. TOS levels need to be increased to limit flow rates and limit oxidation.
System oxidation control on the dual-alkali plant
As explained previously, operation of batch-type Peirce-Smith converters complicates stable chemistry control on a concentrated mode dual-alkali scrubbing circuit. Another factor that is peculiar to the Lonmin operation, and contributes to plant instability, is the smelting profile. Lonmin operates a large furnace (installed at 28 MVA, but rated at 20 MW) that can perform the bulk of its smelting requirements. However, this furnace has experienced a number of planned and unplanned shutdowns since it was designed and built by Hatch in 2002. Three smaller Pyromet furnaces take over smelting responsibility when the large furnace goes down for maintenance, with the effect that overall throughput across the plant falls.
When any furnace is being cooled down or started up, a draught needs to be kept on the furnace. This, coupled with the lower SO2 production, increases the O2/ SO2 ratio. The effect on the dual-alkali plant is notable during these periods, with system oxidation going above design. The formation of fine gypsum crystals in the thickener and the poor crystal growth of calcium sulphite crystals does not allow settling, and the overflow of the thickener becomes milky with fine suspended crystals. Apart from calcium salts carrying over the solution circuit, the removal of solids from the system becomes very difficult due to poor filterability of the small crystals produced.
Lonmin has performed, and is continuing with, trials with flocculent addition in the thickener. Ni et al. (2007) reported the successful trials of flocculent addition to a concentrated mode dual-alkali scrubber. In periods of high system oxidation, flocculation of small crystals will allow filtering and solid removal from the system in order to establish proper system chemistry.
The use of oxidation suppressants (like sodium thiosulphate) can also be considered during times of high system oxidation. Mu et al. (2007) reported that oxidation inhibition efficiency of sodium thiosulphate can range between 85 per cent and 98 per cent (depending on the presence of a catalyst) . The operating cost of the dual-alkali plant will be adversely influenced as a result.
During furnace cooling and furnace start-up periods where no sulphide-containing concentrate is charged, the off-gas from the furnace will be very low in SO2, and could potentially by-pass the dual-alkali plant and be stacked directly. By-pass ducting, valves, dampers, and fans could be included in the design phase.
Product disposal
The decision to install a concentrated mode dual-alkali plant at Lonmin was based on the belief that the product from the plant could be co-disposed with tails from the concentrators to active tailings dams operated by Lonmin.
Lonmin started investigating the possibility of co-disposal soon after the dual-alkali plant was constructed. Two purpose-built mono-disposal dams were used while the investigation was completed. The mono-disposal dams were filled with CaSOx slurry and today re-use options are being considered for the waste in the dams. However, temporary mitigation measures in the form of temporary capping and cut-off trenches to capture any lateral seepage are currently being implemented at a considerable cost.
Due to concerns that were initially raised regarding the potential for contamination of the tailings dam run-off water by leachable species in the CaSOx, as well as the structural integrity of the tailing dam, the decision was taken to trial backfilling in old mine shafts. A backfilling plant with a binder mixing arrangement was built, but after thorough testing for a period of a year, Lonmin decided not to pursue this route. Findings indicated that stability was a concern, and this was related to the variability of the SG of the CaSOx.
During much of the investigation period, the CaSOx product was dispatched to a hazardous landfill site until the Department of Mineral Resources (DMR) granted permission to co-dispose CaSOx with the tailings to an active tailing dam, after an Environmental Management Plan (EMP) Amendment process was undertaken and thorough testing and investigatory work had been completed. Despite the CaSOx comprising less than 1 per cent by weight of the tailing disposal (while being thoroughly mixed), the formation of layering was evident on the tailings dams. De-watering on the dams was influenced negatively, and a decision has been taken not to co-dispose until the phenomenon is properly understood.
Currently Lonmin has no alternative for disposal of the product of the dual-alkali plant other than landfill at a hazardous landfill site. The CaSOx produced in the Lonmin dual-alkali plant is currently rated as hazardous, based on the salt loading. However, the waste has been delisted to be disposed of to a GLB+ site, but variations in the moisture content have frequently resulted in the change of disposal requirements to a hazardous landfill site. Disposal cost is currently more than the combined cost of calcium and sodium usage on the dual-alkali plant.
The impending promulgation of waste legislation and regulations in South Africa will classify slurry with moisture content greater than 40 per cent as a liquid. In the case of the Lonmin CaSOx product, the moisture content is often above 40 per cent, averaging 41.9 per cent. Liquid disposal will be prohibited in the foreseeable future (within 5 years based on the draft regulations), and Lonmin needs to investigate waste minimization alternatives or technologies for the product or waste generated from the dual-alkali plant.
The only alternative to produce a saleable product from the dual-alkali plant, which would then be considered a byproduct and not a waste, is to implement post-oxidation of calcium sulphite to calcium sulphate. Such an oxidation system needs to be on a separate water loop from the main dual-alkali plant and will actually need to function on the back end of an existing concentrated mode dual-alkali plant. Converting calcium sulphite to sulphate will require sulphuric acid addition to lower the pH (to around 5.5) and increase the kinetics of conversion/oxidation. It also requires long residence times (with large tanks) and decent size blowers delivering oxidation air. A second filtering step is necessary for producing a gypsum filter cake. Sodium sulphate will need to be bled from the post-oxidation circuit and will constitute an effluent from such a plant. Lonmin is currently evaluating this option. An important consideration to ensure that this by-product does not become a waste is the selling off of the gypsum, and there seems to be a market for high-quality gypsum in the cement industry in South Africa.
The road ahead
Legislative requirements in South Africa and the rest of the world are continuously becoming more stringent. The South African legislation that has been promulgated and that will be promulgated soon will require that Lonmin (1) increases the efficiency of SO2 capture, (2) captures and evaluates scrubbing of fugitive gases, and (3) produces a saleable product instead of a waste product. Meeting all these requirements, will require significant capital. Based on this, Lonmin decided to do an evaluation and costing exercise of different scrubbing technologies that present a holistic solution to the objectives defined by legislation.
Legislative pressure
Air quality
The National Environmental Management Air Quality Act (No. 39 of 2004) was fully enacted in 2010. The overall approach of NEMAQA, 2004 is to set ambient air quality standards as goals driving emission reduction and emission limits. This is a shift from the point-source based control of emissions to a cause-and-effect approach on the receiving environment.
Ground-level concentrations are specified by the ambient air quality act with maximum SO2 concentrations and a maximum number of times per annum that this level may be exceeded. During a measurement campaign and dispersion modelling exercise, Lonmin determined that fugitive gases from the Pierce-Smith converter need to be captured at an 80 per cent capture efficiency and stacked through the existing concrete stack in order to meet the ground-level concentration limits.
These fugitive gases will constitute a significant contribution to the total tonnage of SO2 vented to the atmosphere in the future (more half of the total tonnage of SO2 that will be vented). As such, Lonmin identified scrubbing of the fugitive gases as the obvious option to target in their total SO2 emission reduction plan. A decision on fugitive gas scrubbing will follow only after monitoring and understanding of the captured fugitive gases.
An amendment to the Air Quality Act was passed in 2009 that sets maximum SO2 concentrations of a point source (stack) . This will require a maximum emission concentration of 1200 mg/Nm3 by the year 2020. For Lonmin, the impact is that the achieved SO2 fixation efficiency needs to improve to almost 99 per cent. This will require an upgrade to the existing dual-alkali plant, or more likely, implementation of tail gas scrubbing on the gas leaving the dual-alkali plant.
The Air Quality Act also specifies that total suspended solids (TSP) concentrations from a point source may not exceed 100 mg/Nm3 by 2015 and 50 mg/Nm3 by 2020. The Lonmin Registration Certificate specifies the availability of the ESP as 98 per cent, which exceeds the availability of the sulphur fixation plant at 97 per cent. This means that the maximum TSP emission needs to be made under sulphur fixation plant (and therefore the venturi scrubber) bypass conditions. Lonmin will need to install a third field on the ESP to achieve the efficiency required by 2015.
Waste management
The Draft Regulations on Waste Classification and Management have been enacted during the second half of 2011 . These regulations state that liquid waste with a moisture content of more than 40 per cent or which liberates moisture under pressure in landfill conditions and has not been stabilized by treatment will be prohibited from being landfill within five years of the enactment. An additional exclusion is for brine or waste with a high salt content (TDS > 5%), which would be prohibited from landfill within eight years.
If one takes into account the waste hierarchy approach that is advocated in the legislation, prevention of waste generation is the preferred option to the treatment of a waste.
Lonmin has adopted this approach and is investigating the production of a by-product from their sulphur fixation technology, which could be sold off. Changes to the dual-alkali plant will require (as a minimum) a post-oxidation circuit to be installed at the back end of the plant, with a new upgraded filter house. This will allow the production of gypsum as the saleable commodity.
Evaluation of other technologies
As part of the current feasibility study, Lonmin will investigate the following options:
Continue to operate the dual-alkali plant while implementing post-oxidation (to gypsum). Combined tail gas and fugitive gas scrubbing to be done in a single Ca-based vessel (Dynawave technology from MECS). Gypsum will be the only saleable product from the plant
Replace the dual-alkali plant with several large vessels that will perform Ca-based scrubbing of the furnace, converter, and fugitive gases. Again, gypsum will be the only saleable product
Replace the dual-alkali plant with an amine absorption circuit (Cansolv Technology from Shell). SO2 can be stripped from the rich amine solution at a controlled rate. This will allow the operation of an acid plant at the back end of the Cansolv plant. Furnace, converter, and fugitive gases to be treated
Replace the dual-alkali plant with a Cansolv plant and amine absorption circuit, but with a SO2 liquefaction plant at the back end.
Lonmin is also conducting a market study for gypsum, acid, liquid SO2, and granular sulphur, and is talking to potential customers.
Conclusion
It is important to understand the impact on the entire value chain when SO2 abatement technologies are evaluated. Each technology will have its own unique benefits and drawbacks, and these should be understood and evaluated in the context of the business requirements, legislative requirements, and the country resource base (availability of energy, water, raw materials, etc).
For a smelter like Lonmin, which does not have sufficient throughput to have a batch Peirce-Smith converter permanently in stack, and that experiences planned and unplanned shutdowns on furnaces, control of the chemistry in the concentrated mode dual-alkali scrubber is difficult. The technology has been experienced as difficult to operate in a stable mode and requires fine control.
Special consideration needs to be taken during design to limit oxidation and establish tight control of the chemistry during inherent plant fluctuations. Equipment design, such as filtering and washing on the filters, is critical to achieve good solids removal and low sodium losses.
The CaSOx product produced from the plant does not have a disposal option that seems to be acceptable in the South African PGM mining context. Special care needs to be taken to allow options for disposal during design of a concentrated mode dual-alkali scrubber.
References
01. ARTHUR, D. Little Inc. 1977. Cambridge, Final Report: Dual Alkali Test and Evaluation Program. EPA-600/7-77-050a-c, vols. I-III. US Environmental Protection Agency, Industrial Environmental Research Laboratory, Research Triangle Park, Durham, North Carolina, May 1977. [ Links ]
02. BEZUIDENHOUT, G.A., EKSTEEN, J.J., and WENDT, W. 2010. Endpoint control in PGM-containing nickel matte converting using flame emission spectroscopy. Processing of Nickel Ores & Concentrates '10, Minerals Engineering International, Falmouth Beach Hotel, Falmouth, UK, 17-18 June 2010. [ Links ]
03. DAUERMAN, L. and RAO, K. 1979 Double alkali process for flue gas desulfur-ization, optimizing for the regeneration of sodium sulfite - Part I: Lime as Regenerant, and Part II: Limestone as regenerant. 72nd Annual Meeting of the Air Pollution Control Association, 24-29 June 1979, Cincinnati, Ohio, NJIT. [ Links ]
04. DREAMEL, D. 1973. Regeneration chemistry of sodium-based double-alkali scrubbing process. EPA-R2-73-186. US Environmental Protection Agency, March 1973. [ Links ]
05. EKSTEEN, J.J. 2010. A mechanistic model to predict matte temperatures during the smelting of UG2-rich blends of platinum group metal concentrates, Minerals Engineering. http://dx.doi.org.ez.sun.ac.za/10.1016/j.mineng.2010.10.017 (in press), [ Links ]
06. EKSTEEN, J.J., VAN BEEK, B., and BEZUIDENHOUT, G.A. 2011. Cracking a hard nut: An overview of Lonmin's operations directed at smelting of UG2-rich concentrate blends. Southern African Pyrometallurgy 2011 International Conference, Cradle of Humankind, South Africa, 6-9 March 2011. Jones, R.T. and den Hoed, P. (eds). The Southern African Institute of Mining and Metallurgy, Johannesburg, 2011. pp. 231-251. [ Links ]
07. LAMANTIA, C.R., LUNDT, R.R., RUSH, R.E., FRANK, T.M., and KAPLAN, N. 1976. Operating experience - CEA/ADL dual alkali prototype system at Gulf Power/Southern Services Inc. Proceedings of the Symposium on Flue Gas Desulphurization, New Orleans, vol. 1, EPA-600/2-76-136a, March 1976. [ Links ]
08. MU, J., WU, Z., CHENG, C., GUAN, B., and ZHAO, W. 2007. Oxidation inhibition of sulfite in dual alkali flue gas desulphurization system. Journal of Environmental Sciences, vol. 19, no. 2, February 2007. pp. 226-231 . [ Links ]
09. NI, W., WU, Z., GUAN, B., LIU., Y, ZHOA, W., and ZHENG, P. 2007. Optimizing flocculation - Sedimentation for regeneration of dual-alkali flue gas desulphurization scrubbing solution. Environmental Progress, vol. 26, no. 3. pp 271-279. [ Links ]
10. TSENG, P.C. and ROCHELLE, G.T. 1986. Calcium sulphite hemihydrate: crystal growth rate and crystal habit. Environmental Progess, vol. 5, no. 1 . pp 5-11. [ Links ]
11. US Environmental Protection Agency. 1980. Summary Report, Sulfur Oxides Control Technology Series: Flue Gas Desulfurization, Dual Alkali Process. EPA 625/8-80-004. October 1980. [ Links ] ♦
Paper received Nov. 2011
Revised paper received Apr. 2012.














