Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 no.6 Johannesburg Jun. 2012
JOURNAL PAPER
Gas-phase extraction of lead and iron from their oxides in a fluidized-bed reactor
L.D. van DykI; E.M.R. MaribaI; Y. ChenI, II; A. JohnsonI; J.H. PotgieterI, III
ISchool of Chemical and Metallurgical Engineering, University of the Witwatersrand, South Africa
IIProcess and Environmental Research Division, Faculty of Engineering, University of Nottingham, Nottingham, UK
IIIDivision of Chemistry & Environmental Science, School of Science and the Environment, Manchester Metropolitan University, Manchester, UK
SYNOPSIS
The extraction of iron and lead from their oxides with a volatile organic ligand such as acetylacetone in the gas phase offers potential advantages of lower energy consumption, recycling of the extractant, recovery of pure metals, and a reduced environmental impact compared with conventional extraction processes. The influence of reaction temperature, ligand flow rate, and metal oxide levels on the extraction and rates of reaction of iron and lead from single metal oxide (synthetic haematite (Fe2O3) and synthetic massicot (PbO)) systems in a fluidized bed was studied. It was found that at the investigated acetylacetone flow rates, the influence of mass transfer was limited, but that the reaction suffered from reactant starvation. When the metal load increased the effect of starvation on the rate of reaction also increased. The reaction kinetics increased with an increase in temperature for both systems. At low metal oxide concentrations more that 80 per cent of the iron and lead could be extracted from their respective metal oxides after only four hours.
Keywords: gas-phase extraction, leaching, iron(III) oxide, lead(II) oxide, acetylacetone.
Introduction
There is currently much debate about the competitiveness of pyrometallurgy, with its high energy consumption and formation of greenhouse gases, as a metal extraction route compared with hydrometallurgy. Unfortunately, hydrometallurgical processes come with their own disadvantages, as they usually comprise conventional leaching, which is non-selective, and subsequently complicated metal recovery processes are required. The final recovery from solution requires additional processing equipment, while the leaching liquor is usually a harsh chemical that is non-recyclable after recovery of the metal and needs to be disposed of in an environmentally friendly way.
Gas-phase extraction of metals is not a new process concept, as many commercial processes have been proposed using a variety of extractants. A few examples include iodine (titanium, zirconium), carbon monoxide (nickel), and hydrogen chloride (tin, copper, and iron). In a gas-phase extraction process one would ideally want to recycle the unreacted extractant or be able to regenerate it after use, something that has been absent from the previously proposed commercial processes1.
In 1985 Cox and co-workers1 proposed a gas-phase extraction process called Selective Extraction and Recovery using Volatile Organic compounds (SERVO). A metal-bearing ore or waste material, which contains a metal present as an oxide, hydroxide, or sulphide, is heated in a fluidized bed and subjected to fluidization by a volatile organic chelating acid and a carrier gas (optional). The extractant reacts with the targeted metal to produce a volatile metal-extractant complex. The product is carried away from the reaction zone and the metal can be recovered, for example, by reduction, while the volatile organic chelating acid is recycled2.3. The SERVO process has been used on a laboratory scale to extract heavy metal contaminants from low-grade ore2, sediment3, spent catalysts, and fly-ash4.
Gas-phase extraction with volatile organic extractants can potentially offer a solution to the limitations of conventional metal extraction. Duke2 has shown that very selective volatile organic chelating extractants can be developed for specific applications.
Even if several volatile-metal complexes are formed, a series of temperature-controlled condensers can be used to produce clean gas streams as these metal complexes have different crystallization and condensation temperatures4,5. The acidic extractant can be regenerated and the elemental metal obtained if it is thermodynamically feasible to use hydrogen as a reducing agent. Currently, the literature on the reaction mechanisms and extraction kinetics of laboratory-scale studies is limited.
In order to obtain a better understanding of the reaction kinetics of the gas-phase extraction process, our group has been studying the influence of the reaction conditions on the extraction of metals from their oxide forms with acetylacetone. Some of the results for the extraction of single metals from a mixture of synthetic oxide (haematite (Fe2O3) or massicot (PbO)) and silica sand are presented in this paper.
Iron and lead extraction
The extractions of iron or lead from their minerals both follow pyrometallurgical routes. Pyrometallurgical extraction makes it difficult to extract these metals from low-grade sources. Concentration of the metal precursor material is usually required. Large amounts of CO2 and SO2 are released into the atmosphere, and the process requires large amounts of energy. The global demand for lead and iron is increasing and reserves are being consumed. Lead is used extensively in lead-acid batteries, which are seen as green-energy alternatives to some fuels. This study considers the use of acetylacetone as an organic extractant to extract lead and iron from their oxide forms. As gas-phase extraction in a fluidized bed offers good solid-vapour contact, it is expected that this technology can be applied directly to some low-grade sources of both metals. For example, iron can be extracted from sources such as spent iron oxide catalyst, coal mine sludge, tailings that contain a significant amount of iron, and red mud (waste stream from the Bayer process) 6.
The reaction of haematite and acetylacetone is given by the following equation:
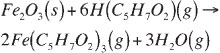
Iron(III) acetylacetone (Fe(C5H7O2)) melts at 183°C and volatilizes over a range of temperatures (92-275°C)7. Previous studies using Fe2O3 have shown that iron readily reacts with acetylacetone3.
No previous study could be found in the literature on the extraction of lead from massicot with acetylacetone in the vapour phase. This reaction equation can be written as follows:

The melting point of lead(II) acetylacetonate (Pb(C5H7O2)) is 142°C and the volatilization temperature range is not available in open literature. Figure 1 represents a generic commercial gas-phase extraction process using acetylacetone as the extractant, with lead being the targeted metal.

Acetylacetone is vapourized at its boiling point (140°C) and passed through a heated bed of lead-bearing material. The flux of the acetylacetone vapour fluidizes the bed and the reaction takes place, forming a volatile lead(II) acetylace-tonate (Pb(acac)2). The reaction products (Pb(acac)2 and H2O) and unreacted acetylacetone leave the bed and enter a crystallizer where the liquids are separated from the lead(II) acetylacetonate. The water and the acetylacetone are separated by distillation (boiling point difference of 40°C) and the acetylacetone recycled back to the reactor. Lead(II) acetylactonate can be sold as is, or subjected to reduction or decomposition to form lead and acetylacetone, which will be recycled back to the reactor. A similar process can be developed to recover iron from low-grade iron sources.
Experimental procedure
Materials
The bed material consisted of a mixture of silica sand and synthetic haematite (99% Fe2O3) or synthetic massicot (99% PbO) with a total mass of 50 g. The particle sizes of the silica sand and the metal oxides ranged from +53 μm to -75 μm The fluidization gas was acetylacetone (> 99% CH3COCH2 COCH3) at temperatures in excess of its boiling point (140°C).
The minimum fluidization velocities (umin) were calculated at the lowest experimental temperatures and at the highest metal oxide levels. The calculated values represent the highest minimum fluidization velocities at the experimental conditions. Gas viscosity increases with temperature, the density of the gas decreases with temperature, and solid density increases with metal oxide levels. The minimum fluidization velocity of the Fe2O3 bed is 0.00071 m/s and for the PbO bed is 0.00071 m/s. These values are below the experimental linear velocities of the acetylacetone vapours.
Experimental setup
The experiments were carried out in a laboratory-scale glass fluidized-bed reactor (15 mm ID and 50 cm high) equipped with a porous glass distributor. A 500 mL round-bowl flask evaporator was located underneath the column and heated by a heating mantle with temperature control. The temperature of the fluidized-bed reactor was measured with a type-K thermocouple located in the middle of the column, and the temperature was controlled with a proportional-integral-differential (PID) controller. The column was heated with heating wire and insulated with ceramic wool to prevent heat losses to the environment. A condenser was connected to the top of the column to condense the vapour product, which was dissolved in a solvent. A detailed description of the experimental apparatus can be found in van Dyk et al.8
Procedure
The influence of the bed composition, vapour flow rate, and reaction temperature on the extraction kinetics were investigated. The fluidized-bed reactor was charged with three different weight percentages (1, 3, or 10 wt %) of Fe2O3 or PbO. The reaction temperature was varied between 190°C and 250°C for the Fe2O3 mixture, and between 200°C and 300°C for the PbO mixture. The acetylacetone flow rate was fixed at 1 mL/min or 3 mL/min at 25°C. The exit gas stream, consisting of the volatized metal-acetyacetonate and the unreacted ligand, was collected from the top of the fluidized-bed reactor in a condenser connected to a bubbler filled with ice-cooled ethanol. The product samples were collected at regular time intervals and analysed for iron or lead content with atomic absorption spectrometry (AAS). The AAS results were used to calculate the extraction with time.
Results and discussion
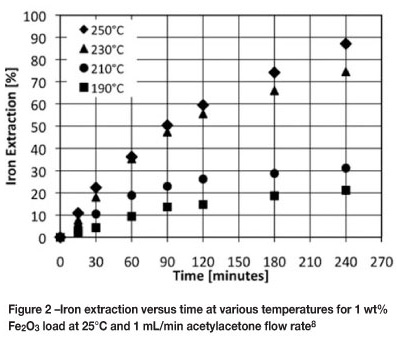
The influence of the reaction temperature on the extraction kinetics of synthetic haematite (Fe2O3) and synthetic massicot (PbO) was examined. Iron extraction results are presented for a 1 wt% and a 10 wt% Fe2O3/silica mixture at various temperatures (Figure 2 and Figure 3). For both metal oxide loads the extraction of iron increased with increasing reaction temperature. The influence of temperature on the extraction and rate of reaction is higher at lower Fe2O3 load.
For 1 wt% Fe2O3 load (Figure 2), 87 per cent of the available iron was extracted after 4 hours at 250°C. As the temperature increases the initial reaction rate (gradient of curve) increases, with the biggest increase between 210°C and 230°C. The influence of temperature on the initial reaction rate (up to 120 minutes) above 230°C is much lower, as extraction at 250°C is very similar to that at 230°C. The overall kinetics of a gas-solid reaction system may be influenced by gas-phase mass transfer of the reactants or products, pore diffusion, and chemical kinetics (including adsorption/desorption). Mass transfer involves gaseous diffusion through a boundary layer, which is not an activated process and consequently the influence of temperature on a mass-transfer controlled process is limited. At the reaction conditions presented the gas velocity (0.036 < u < 0.044 m/s) is high and the particles are small (diameter ranges between +53 μm and -75 μm), and the reaction conditions are ideal for studying chemical reaction kinetics9. In general it can be seen that the rate of reaction decrease as the reaction progress with time. As time progress the particle is consumed by the reaction, and 'shrinks' as the reaction products are removed from the particle and its surface area decreases. The gas-solid reaction takes place on the surface of the particle and as the particle shrinks, the surface available for reaction decreases, slowing down the reaction.
For 10 wt% Fe2O3 load, the influence of temperature on the reaction kinetics is less significant than for the 1 wt% system and the extraction appears to be linear. Even though only 31 per cent of the iron is extracted from a 10 wt% Fe2O3 load compared with 87 per cent for 1 wt% at 250°C, 1 mL/ min after 4 hours, the average extraction rate over the four-hour period is 4.5 mg Fe per minute compared with 1.27 mg Fe per minute. In the case of a 10 wt% Fe2O3 load, the surface area available for reaction is greater than that of a 1 wt% Fe2O3 load as more particles are present (particle sizes similar). It can therefore be said that as the available surface area of the solids increases, the more efficiently acetylacetone is used. The extraction stays linear with time, even though the available surface area of the particles decreases. As with the 1 wt% Fe2O3 load, it is expected that the reaction rate will decrease with time, although this is not seen here. A discussion on this matter will follow.
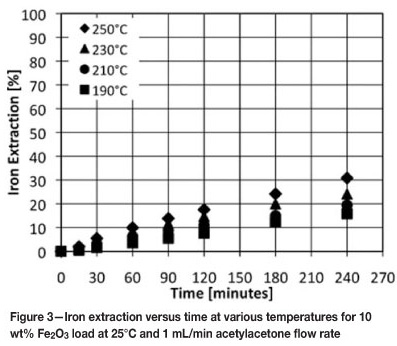
The extraction of lead from a 1 wt% PbO load at various temperatures and 1 mL/min acetylacetone flow rate is presented in Figure 4. The influence of temperature on the extraction of lead is similar to that on an 1 wt% Fe2O3 load. It can be seen that there is a large increase in the initial extraction rate of lead between 250°C and 300°C. At 300°C, 78 per cent lead was extracted from a 1 wt% PbO/silica mixture after 4 hours at 1 mL/min acetylacetone flow rate. The extraction of lead at 250°C and 300°C is lower than that of iron for a similar metal oxide load and acetylacetone flow rate. Cox, Cottrell, and Youssif examined the use of various β-diketones (acetylacetone is a β-diketone) for metal extraction, and found that these reagents reacted preferentially with iron(III). The formation constants of M(III) compounds tend to be larger than those of M(II) compounds, where M denotes metal1. The extraction results obtained for lead at 250°C and 300°C are consistent with their findings that acetylacetone reacts preferentially with iron(III). What is interesting to note is that the extraction of lead at 200°C is higher than that of iron at 210°C. This does not agree with the previous observations. A possible explanation for these results is that diffusion of the product through pores may play a role in the overall extraction kinetics at lower temperatures. Lead(II) acetylacetonate (vapour pressure 27.9 Pa at 120°C) is more volatile than iron(III) acetylacetonate (vapour pressures 14.6 Pa at 120°C), and it could be said that diffusion of the product will be faster for the lead system10. This will, however, have to be proven with additional experimental work.
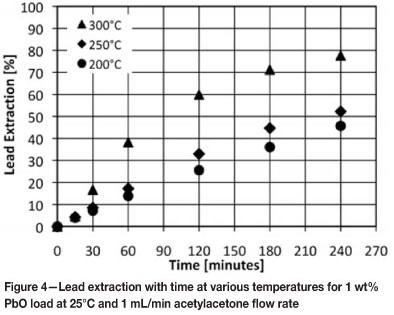
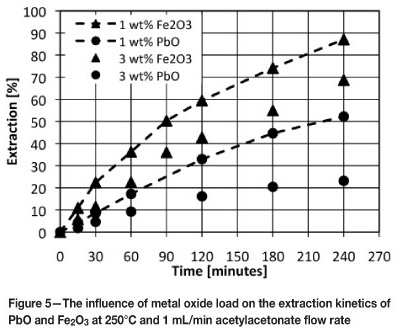
Figure 5 compares the extraction of lead and iron from their respective oxides at 250°C and 1 mL/min acetylacetone flow rate at 1 and 3 wt% metal oxide loads. It can also be seen that extraction decrease with an increase in initial metal oxide load. The influence of metal oxide load is greater for lead than for iron.
In order to explain these results, additional tests were conducted where the acetylacetone flow rate was varied. The results for 1 wt% PbO at 250°C are presented in Figure 6. Similar results were obtained for the Fe2O3 system and have been reported elsewhere8. By increasing the acetylacetone flow rate an increase in the reaction rate and in the amount of lead extracted is achieved. For a 1 wt% PbO load at 250°C, the extraction increases from 52 per cent to 88 per cent after 4 hours.

The acetylacetone flow rate influences the concentration of the extractant, the availability of the extractant, and the degree of turbulence (linear gas velocity) in the fluidized bed. An increase in the concentration and availability of the acetylacetone has an influence on diffusion (function of concentration difference) and on the chemical reaction. An increase in the degree of turbulence influences the external mass transfer of the acetylacetone from the bulk solution to the metal particle. In our experiments an increase in the volumetric flow rate of acetylacetone increased the linear gas velocity (0.036 m/s to 0.119 m/s) as the same column was used for all the experiments. Szekely et al.9 states that if gas-solid reactions are carried out with identically sized particles at the same temperature and the reaction shows a dependence on the linear gas velocity, this indicates that external mass transfer plays an important role. The results in Figure 6 therefore suggest that mass transfer plays an important role in the overall reaction kinetics. However, it has been shown previously that adding a carrier gas, for example nitrogen, has little influence on the rate of reaction at similar acetylacetone flow rates8. This then means that an increase in the linear gas velocity accompanied by a decrease in the acetylacetone concentration has no effect on the extraction and that an apparent dependence on the gas velocity exists.
This can be the case only if the reaction suffers from reactant starvation. When a sufficient amount of the reactant is consumed by the solid, the gaseous reactant in contact with the solid is at a concentration lower than the bulk concentration and the effective driving force of the reactant will depend on the gas flow rate. This also explains why at 10 wt% Fe2O3 load the rate of extraction is lower than at 1 wt% Fe2O3 load, even though the available surface area for reaction is greater. In another study on the extraction of aluminium within our group, it has been seen that there is an optimum extractant flow rate after which an increase in extractant flow rate has no influence on the rate of reaction11. When the reaction suffers from starvation, an increase in metal oxide load has to be accompanied by an increase in acetylacetone flow rate to achieve similar conversions at the same reaction time and temperature as for lower metal oxide loads.
From the results presented it can be seen that similar extractions (88 per cent and 87 per cent) can be obtained from a PbO and Fe2O3 system at 250°C for 1 wt% load, if the acetylacetone flow rate for the lead system is increased to 3 mL/min.
Conclusions
It has been shown that gas-phase extraction can be used for iron extraction from synthetic haematite (Fe2O3) and lead extraction from synthetic massicot (PbO) using acetylacetone as a volatile organic chelating extractant. The reaction rate of both reactions is temperature-dependent, but differs for the two metal oxide species. At the acetylacetone flow rates investigated the reaction suffered from reactant starvation, which influenced the rate of reaction to a large extent. This influenced the conclusions that could be drawn from the effect of the metal oxide load on the extraction and extraction rates. An in-depth study will have to be performed at the investigated reaction conditions without any reactant starvation to determine the overall rate- controlling step/s. With the gas-phase extraction process, 87 per cent of the iron could be extracted from a 1 wt% synthetic Fe2O3 mixture after 4 hours at 250°C and 1 mL/min acetylacetone, while 88 per cent of the lead could be extracted from a 1 wt% synthetic PbO mixture at similar reaction conditions, but using 3 mL/min acetylacetone. The extraction results presented in this paper are limited to single metal extraction, and future work will focus on ore systems to study the influence of minerals and phases on the extraction kinetics.
Acknowledgements
The authors would like to thank the National Research Foundation of South Africa for its financial assistance through the Thuthuka Programme.
References
1. Cox, M., Duke, P.W., and Gray, M.J. Extraction of metals by the direct thermal attack of organic reagents. Proceedings of Extraction Metallurgy '85. Institute of Mining and Metallurgy, London, 1985. pp. 33-42. [ Links ]
2. Duke. P.W. Thermal stability and reactivity of metal extraction coordination compounds. MPhil Thesis, University of Hertfordshire, UK, 1985. [ Links ]
3. Allimann-Lecourt, C., Bailey, T.H., Cox, M., Gilby, L.M., and Robinson, J. Extraction of heavy metals from sediments using the SERVO process. Land Contamination and Reclamation, vol. 7, no. 4, 1999. pp. 265-269. [ Links ]
4. Allimann-Lecourt, C., Bailey, T.H., and Cox M. Purification of combustion fly ashes using the SERVO process. Journal of Chemical Technology and Biotechnology, vol. 77, 2002. pp. 260-266. [ Links ]
5. Berg, E.W. and Hartlage, F.R. Fractional sublimation of various metal acetylacetonates. Analytica Chimica Acta, vol. 33, 1964. pp. 173-181. [ Links ]
6. Apblett, A.W. and Barber, K. Green technology of extraction of iron from ores and other materials. Advances in Material Science and Environmental and Nuclear Technology, vol. 222, 2010. pp. 168-176. [ Links ]
7. Potgieter, J.H., Kabemba, M.A., Teodorovic, A., Potgieter-Vermaak, S.S. and Augustyn, W.G. An investigation into the feasibility of recovering valuable metals from solid oxide compounds by gas-phase extraction in a fluidised bed. Minerals Engineering, vol. 19, 2006. pp. 140-146 [ Links ]
8. Van Dyk, L., Mariba, E.R.M., Chen, Y., and Potgieter, J.H. Gas-phase extraction of iron from its oxide in a fluidized bed reactor. Minerals Engineering, vol. 23, no. 1, 2010. pp. 58-60. [ Links ]
9. Szekely, J., Evans, J.W., and Sohn, H.Y. Gas-Solid Reactions. Academic Press, New York, 1976. [ Links ]
10. Igumenov, I.K., Basova, T.V., and Belosludov, V.R. Volatile precursors for films deposition: vapor pressure, structure and thermodynamics. Mizutani, T. (ed). Application of Thermodynamics to Biological and Material Science. InTech, Croatia, 2011. pp. 521-526. [ Links ]
11. Mpana, N. The gas-phase extraction of aluminium. Research report, School of Chemical and Metallurgical Engineering, unversity of the Witwatersrand, Johannesburg, South Africa, 2011. [ Links ]
This paper was first presented at the, Industrial Fluidization South Africa Conference, 16-17 November 2011, Cradle of Humankind, South Africa.














