Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 n.5 Johannesburg May. 2012
TRANSACTION PAPER
Treatment of Cr(VI)-containing wastes in the South African ferrochrome industry - a review of currently applied methods
J.P. BeukesI; P.G. van ZylI; M. RasII
IChemical Resource Beneficiation, North-West University, Potchefstroom Campus, Potchefstroom, South Africa
IIGravmax (Pty) Ltd, South Africa
SYNOPSIS
South Africa holds approximately three-quarters of the world's viable chromite ore resources and dominates the global production of ferrochrome. Albeit completely unintended, small amounts of Cr(VI) are formed during ferrochrome production. Certain Cr(VI) species are regarded as carcinogenic, hence making the treatment of some ferrochrome waste materials necessary. In this paper, the Cr(VI) treatment strategies currently employed by the South African ferrochrome producers are investigated by means of a literature review and a questionnaire survey. From the discussion, it is evident that various treatment strategies are available to deal with Cr(VI)-containing waste in the ferrochrome industry. However, by far the most commonly applied treatment strategy remains the aqueous reduction of Cr(VI) with ferrous iron. The advantages and the correct application of this strategy, together with the disadvantages and pitfalls, are argued. Innovative improvements on historic practices are also discussed
Keywords: hexavalent chromium treatment, Cr(VI) treatment, ferrochrome production, Cr(VI) containing waste, South Africa
Introduction
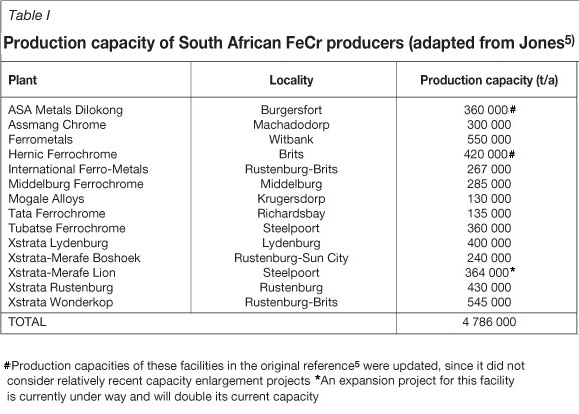
South Africa holds approximately three-quarters of the world's viable chromite ore resources1,2,3 and dominates the global production of ferrochrome(FeCr)4. FeCr is a relatively crude alloy of predominantly iron and chromium, used mainly in the production of stainless steel. There are currently fourteen separate FeCr smelters in South Africa, with a combined production capacity5 in excess of 4.7 Mt/a. Table I provides an overview of the production capacities of these facilities and also indicates recent capacity increases. Although the current electricity shortage in South Africa and the rising cost of power have partially stunted growth in this electricity-intensive industry, it is foreseen that South Africa will remain the leading producer of FeCr in the foreseeable future.
Hexavalent chromium, Cr(VI), is formed in small quantities as an unintended by-produc during ferrochrome production. Certain Cr(VI) species are regarded as carcinogenic, with specifically airborne exposure to these Cr(VI) species being associated with cancer of the respiratory system7,8. In a recent paper, the generation of Cr(VI) in the various production processes utilized by the South African FeCr industry was reviewed and possible mitigating steps were discussed6. In the present paper, Cr(VI) treatment strategies currently employed by the South African FeCr producers in dealing with waste products, possibly containing Cr(VI), are reviewed.
Questionnaire survey
This paper is primarily a review and not an empirical study. However, the knowledge of the authors and the information in the public domain were augmented by a survey questionnaire pertaining to Cr(VI) treatment strategies. This survey was circulated to individual South African FeCr smelters in 2011. The questionnaire was kept simple to enhance participation - mostly requiring the respondent to tick the most appropriate answer box, with space for additional comments or notes. The questionnaire consisted of seven questions, which are summarized as follow:
Q1: Assessing whether any Cr(VI) treatment took place onsite
Q2: Determining the process origin(s) of the possible Cr(VI) containing material that is treated on site
Q3: Whether aqueous or direct treatment(s) of dry materials, possibly containing Cr(VI),were used
Q4: If aqueous Cr(VI) treatment took place, which reducing agent(s)was/were used
Q5: If aqueous Cr(VI) treatment took place, why the specific reducing agent(s) was/were chosen.
Q6: If aqueous Cr(VI) treatment took place, what was the pH range of the process or waste water
Q7: What analytical technique(s) was/were used to assess Cr(VI) levels.
Of the fourteen FeCr smelters mentioned previouslys, thirteen are full-time FeCr smelters and one is a part-time FeCr smelter. Nine of the full-time FeCr smelters (~70%) completed the survey. The results from this survey are included in the discussions that follow.
Cr(VI) treatment
Ma et al.9 reported on the formation, treatment, and stabilization of certain South African metallurgical wastes and pointed out that there are a number of different methods to deal with these wastes:
Minimization of the wastes at the source by optimizing the operational parameters
Direct recycling of certain materials to the furnace
Recovery processes, which include hydrometallurgical methods and pyrometallurgical methods
Solidification/stabilization methods, for instance cementation and vitrification (glassification) processes
Use as a raw material in an appropriate product, such as fertilizer
Treatment and land filling.
According to the authors, most of the above-mentioned treatment options are used at least to some degree in the South African FeCr industry on possible Cr(VI)-containing wastes. Minimization of the wastes at the source by optimized operational parameters is a prime objective of all South African FeCr producers, since minimized waste implies higher profitability. Direct recycling is also applied. However, direct recycling of furnace off-gas wastes (e.g. bag filter dust and scrubber sludge) to a FeCr smelting furnace could lead to the build-up of more volatile species, such as sodium and zinc9, resulting in lower production capacity and even possibly the risk of explosions. Recovery processes used by
South African FeCr producers to recover valuable Cr units from wastes are currently limited mainly to coarser materials, such as FeCr slag10-14. Solidification/stabilization of FeCr wastes is also currently mostly limited to slag, since some South African FeCr slags have recently been declassified, making it possible to utilize these slags as agglomerate material in commercial cementation applications. Bag filter dust and scrubber sludge are still classified as hazardous wastes, therefor very little of these materials are treated in this manner, although in theory it is possible to achieve solid stabilization of Cr(VI)-containing wastes15-17. Apart from FeCr slag, the only other FeCr-related waste that is utilized as a commercial product is relatively small quantities of a calcium-rich waste produced by a specific smelter. This is utilized as a soil additive or fertilizer. However, by far the most common process for dealing with possible Cr(VI)-containing waste in the South African FeCr industry is aqueous chemical Cr(VI) reduction, with subsequent precipitation of the Cr(III) hydroxides and land filling in specially designed waste facilities. In the survey conducted, all the respondents indicated that aqueous Cr(VI) treatment is performed on site. Materials most commonly treated include bag filter dusts, scrubber sludge, and certain process waters.
As indicated by Beukes et al.6, Cr(VI) can be generated during various FeCr production processes. By volume, slag is the main waste material generated6. However, with regard to Cr(VI) content, fine particulate matter originating from the off-gas of high-temperature processes can be regarded as the most significant Cr(VI)-containing waste material generated by the FeCr industry6,18. Exposure to airborne Cr(VI) by inhalation is also much more hazardous than other exposure routes7, which further emphasises the importance of these fine, potentially airborne materials.
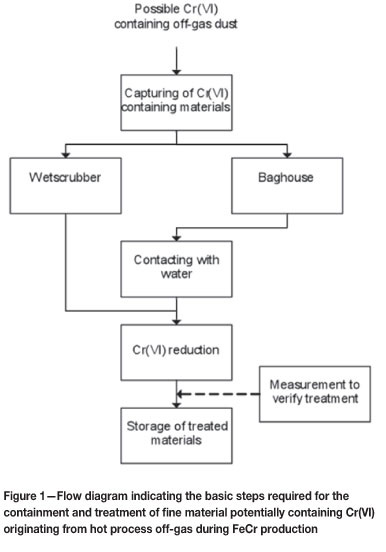
In order to treat Cr(VI) wastes effectively, several basic process steps have to be followed. These include capturing materials that potentially contain Cr(VI) (if the waste originated from an off-gas), contacting such materials with water, reducing Cr(VI) to Cr(III) (e.g. in the aqueous phase) and storing the treated material. These treatment steps are illustrated in Figure 1, as applicable to possible Cr(VI)- containing wastes originating from process off-gas streams. These steps seem very elementary; however, there are certain pitfalls that must be avoided. The first three steps, i.e. i) capturing of fine materials from off-gases, ii) contacting the contained materials with water, and iii) Cr(VI) reduction to Cr(III), are therefore discussed in more detail. Analytical verification of the effectiveness of the treatment and storage of the treated materials are not considered in this paper.
Capturing fine materials potentially containing Cr(VI) from off-gas streams
Gas cleaning equipment currently used by the South African FeCr industry for processes generating fine particulate matter are typically wet venturi scrubbers and bag filters. Bag filters are associated mainly with semi-closed furnaces and certain other processes, such as milling, agglomeration and curing of the agglomerates, while wet scrubbers are associated mainly with closed furnaces6,18.
In general, it can be stated that wet venturi scrubbers should be regarded as a better control mechanism for removing Cr(VI)-containing particulate matter from off-gas than bag filter systems. This belief stems from the fact that wet scrubbers immediately contact the particulate matter possibly containing Cr(VI) with water during the capturing mechanism. This is in contrast to bag filter systems, which capture the particulates as dry matter. As mentioned previously, airborne Cr(VI) is more hazardous than aqueous Cr(VI)7. However, it is not that straightforward to recommend the use of wet scrubbers for all FeCr production processes in the South African FeCr industry. South Africa has a relatively low and unpredictable rainfall. On average, South Africa only receives approximately 480 mm/a, which is about half of the 860 mm/a world average19. The Bushveld Igneous Complex, where all the chromite reserves in South Africa are located, lies within a semi-arid region. According to a 37-year rainfall record from the South African Weather Service20, Burgersfort, a town situated on the eastern limb of the Bushveld Igneous Complex, has an annual average rainfall of 493 mm/a. During this period, it had a minimum annual rainfall of 163 mm/a, while a maximum of 1005 mm/a was measured. For Brits and Rustenburg, towns situated on the western side of the Complex, rainfall averages of 627 mm/a (50-year average) and 538 mm/a (17-year average) were reported, respectively. The minimum rainfall values for Brits and Rustenburg were 255 and 274 mm/a, respectively, while the respective maximum values reported were 1362 and 954 mm/a. Therefore, it is clear that rainfall, which has a direct effect on the availability of surface water, is relatively low and unpredictable in the Bushveld Igneous Complex. Although process water is usually clarified and reused in a typical wet venturi scrubbing process, large quantities of water are lost due to the unavoidable evaporation by the hot off-gas that is cleaned. As a result, the use of wet scrubbing as a process technique is sometimes unpractical and could in some cases even be prohibited by South African environmental legislation (e.g. through overall environmental considerations during an Environmental Impact Assessment). The water requirements of communities, agriculture, and livestock will always take preference. In industry, the use of wet venturi scrubbers is usually limited to applications where carbon monoxide (CO) rich off-gas can be obtained and latent energy recovered from the subsequent combustion of such off-gas21. Other technical process-related aspects, as well as the capital and operational costs of the different off-gas cleaning technologies, are obviously also taken into consideration when off-gas cleaning technologies are chosen for a particular application.
Contacting the captured materials with water
Since the relative health risk associated with exposure to airborne Cr(VI) is much higher than the risk associated with exposure to aqueous Cr(VI)7, it is essential to contact the captured particulate matter with water as soon as possible. This simple action is extremely effective in mitigating possible occupational health impacts of Cr(VI).
Wet scrubbers immediately contact captured off-gas particulates with water; however, bag filter units do not. When considering a generic bag filter at a FeCr smelter, the following procedure is recommended. The closed hoppers into which the dust from the bag filters falls should be sealed off at the bottom with a rotary or double flap valve. These valves should release the dry dust from the hopper at a controlled rate. The discharge from each valve should fall into a sealed chute leading to a furrow of running process water directly below. This furrow should also be covered with removable cover plates to prevent any possible wind dispersal. The abovementioned procedure (or a similar procedure) will ensure that dry captured dust is contacted with water as soon as possible, preventing dry dust spillages or wind dispersal. The Cr(VI) present in this process water or sludge can then be reduced as described below.
Cr(VI) reduction
There are numerous reducing agents that can be utilized to convert Cr(VI) to Cr(III) in the aqueous phase. However, within the context of this paper, the reduction of Cr(VI) with Fe(II) in the aqueous phase warrants an in-depth discussion, since all respondents indicated in the survey that they used aqueous ferrous treatment to reduce Cr(VI) to Cr(III). The reasons why ferrous chemicals, such as ferrous chloride or ferrous sulphate, have been the reducing agents of choice for South African FeCr producers are:
Ferrous iron is an inorganic reducing agent that leads to the formation of insoluble Cr(III) hydroxide species in the pH range that is applicable to the FeCr production process and waste waters. This might seem inconsequential, but it is well known that most organic compounds can reduce Cr(VI)22. However, some organic compounds can form water-soluble Cr(III)-complexes that are undesirable. Although soluble Cr(III) species are not toxic or carcinogenic, soluble Cr(III) could be transported by ground or surface water and come into contact with manganese dioxide - a naturally occurring oxidant for Cr(III)23,24,25. Therefore, Cr(VI) might be formed far from the original source if an inappropriate organic reducing agent is used
Ferrous reduction of Cr(VI) has received much research attention26-34 and the theory of reduction is therefore well understood
In the survey, numerous South African FeCr producers indicated that this treatment strategy is used since it is considered a proven technology
Ferrous iron reduction is effective over the entire pH range applicable to FeCr process or waste waters (pH values between 6.2 and 9.0 were reported in the survey). This is in contrast to other industrially utilized inorganic Cr(VI) reducing species, such as S(IV) (dissolved SO2, sulphite, or bisulphite), which can be used effectively only at pH < 535,36. Dissolved O2 can oxidize Fe(II), especially under alkaline conditions29,32,34. This competing reaction can reduce the effective pH range of ferrous iron reduction of Cr(VI). However, the oxidation of Fe(II) by dissolved O2 will not affect the efficiency of Cr(VI) reduction, or result in unnecessary losses of Fe(II), if the conditions are turbulent enough at the Fe(II) dosing point. Buerge and Hug32 compared the rate of reduction of Cr(VI) by Fe(II) to the rate of oxidation of Fe(II) by dissolved O2. They reported that Cr(VI) reduction by Fe(II) was faster than Fe(II) oxidation of dissolved O2 by the factors of 3x104, 6x103 and 1x103, measured at pH 4, 6, and 8 respectively. He et al.29 proved that Fe(II) is an effective reducing agent for Cr(VI), even at hyper-alkaline conditions, if enough turbulence is achieved to ensure almost instantaneous mixing. However, if Fe(II) is added to relatively stagnant process or waste water, only the Cr(VI) immediately contacted with the Fe(II) will be reduced and the rest of the Fe(II) will be oxidized to Fe(III) without coming into contact with Cr(VI). Typically, a furrow with running process water, a turbulent pump sump, or an agitated mixing tank could be considered as suitable localities to dose ferrous iron for Cr(VI) reduction, while dams, clarifiers/thickeners, and other relatively stagnant water bodies would be inappropriate dosing points
Ferrous chemicals are readily available in South Africa. More than half of the survey respondents indicated that availability of ferrous reducing agents has been a key factor when selecting a reducing agent for aqueous Cr(VI).
Although highly effective in reducing Cr(VI) to Cr(III), the use of ferrous chemicals has numerous disadvantages. These include:
Their use increases the total dissolved solids (TDS) content of the process and waste water. Fe(II) is removed by oxidation to Fe(III), which consequently forms an Fe(III) hydroxide. This hydroxide precipitates from solution at the pH levels relevant to the FeCr process and waste waters. However, the chloride or sulphate remains in solution, causing the increase in TDS
The abovementioned increase in TDS could result in increased scale build-up in pipes, spray nozzles of wet scrubber systems, and other equipment. This ultimately results in increased downtime and therefore production losses
Although the 'major' environmental and health risk, i.e. Cr(VI), is effectively dealt with during the reduction of Cr(VI) to Cr(III) by Fe(II), the increased TDS and chloride or sulphate load could result in increased salination of surface and ground water, due to potential process and waste water leakages. Although salination of surface and ground water is not regarded as serious as Cr(VI) contamination, it is certainly not acceptable
Since aqueous Fe(II) is oxidized by dissolved O2 at elevated pH levels29,32,34, ferrous chemicals have to be stored prior to use as strong acid solutions (e.g. ferrous chloride) or as solid powders (e.g. ferrous sulphate). Acid solutions of ferrous chloride are regarded as hazardous chemicals. FeCr producers therefore usually store ferrous chloride solutions in special tanks in bunded areas. These bund walls should be able to contain spillages that might occur and thereby prevent pollution of the environment and/or prevent injuries to personnel. Notwithstanding these safety measures, ferrous chemicals are considered occupational health risks for the operational personnel at FeCr smelters
Ferrous chemicals have to be transported by road from the manufacturers to the user, i.e. the FeCr producer. This results in additional traffic on the South African roads, which are already regarded as relatively overloaded and dangerous.
Theoretically, the electrochemical reduction of Cr(VI) is a feasible solution to the abovementioned problems, since the ferrous ions are generated in situ and are not associated with chloride or sulphate anions. The electrochemical process occurs through redox reactions taking place at the surface of conductive iron electrodes immersed in water, leading to the subsequent reduction of Cr(VI) by Fe(II)37:
Oxidation reaction at the anode:

Reduction reaction at the cathode:
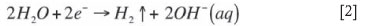
Reaction stoichiometry with Cr(VI):

Although the electrochemical reduction of Cr(VI) has received considerable research attention37-54, the needs of the FeCr industry have until recently not really been addressed. The reason for this is that FeCr process and waste water have not been the intended treatment objective of most of these studies. However, the development of proprietary electrochemical Cr(VI) treatment technology in South Africa55 has recently resulted in the full-scale implementation of electrochemical reduction of Cr(VI) in process and waste water at several local ferrochrome producers. Approximately 45 per cent of the survey respondents indicated that this specific electrochemical reduction technology was already being used on site, while additional respondents indicated that they were considering this treatment option. However, it is of concern that no peer-reviewed public domain information on the effectiveness and environmental soundness of this treatment process is available, and it can therefore not be compared to the currently applied chemical Fe(II) reducing process.
This absence of public domain information on the abovementioned technique is indicative of the reduced interest in the research and development field over the last decade by the South African FeCr industry at large. Previously, some South African ferroalloy companies had separate research and development (R&D) departments, which supported research initiatives. However, an increasing focus on only the core business activities has led to most of these in-house R&D initiatives being terminated or substantially reduced. Additionally, the R&D efforts have not been transferred to external/contract-based R&D organizations. This has in part led to other, potentially more environmentally friendly Cr(VI) treatment options being ignored. One such option is the bacterial reduction of Cr(VI), which was piloted by the South African ferrochrome industry56, but never fully implemented. The implementation of such alternative Cr(VI) treatment options might require more precise control of process parameters (e.g. water temperature, nutrient levels, etc.) and possible support from R&D personnel.
Conclusions
From the literature review, it would seem that a number of treatment options for Cr(VI)-containing waste are feasible. However, all the survey respondents indicated that the aqueous reduction of Cr(VI) with ferrous iron was currently the preferred method. This treatment option is a proven technology that is well researched, and the reducing agents (e.g. ferrous chloride or sulphate) are readily available in South Africa. However, this treatment strategy has some disadvantages, which should be considered by FeCr producers. The actual Cr(VI) reduction step should also not be considered in isolation, since the capturing of possible Cr(VI)-containing materials (e.g. capturing of fine particulate matter with bag filters or scrubber systems) and contacting these materials with water as soon as possible are equally important within the overall Cr(VI) treatment strategy. Waterborne Cr(VI) is much less hazardous than airborne Cr(VI), and consequently the correct application of these simple steps will result in a significant decrease in the overall occupational health and environmental risks associated with Cr(VI) at a FeCr smelter.
It also seems that the ever-growing environmental consciousness of the South African FeCr producers has resulted in the implementation of in situ electrochemically-generated ferrous reduction of Cr(VI). Almost half of the South African FeCr producers have already implemented this technology. Theoretically, electrochemically-generated ferrous reduction of Cr(VI) has the potential to negate most of the negative aspects associated with the traditional chemical reduction of Cr(VI). However, no public domain information on the effectiveness and environmental soundness of this technology is currently available, making comparison with more traditional chemical Fe(II) reduction impossible.
Acknowledgements
The South African ferrochrome producers who participated in the voluntary survey are thanked for their contribution. Also, the South African Weather Service is acknowledged for supplying the rainfall data.
References
1. MURTHY, Y.R., TRIPATHY, S.K., and KUMAR, C.R. Chrome ore beneficiation challenges and opportunities - A review. Minerals Engineering, vol. 24, 2011. pp. 375-380. [ Links ]
2. CRAMER, L.A., BASSON, J., and NELSON, L.R. The impact of platinum production from UG2 ore on ferrochrome production in South African. Journal of the South African Institute of Mining and Metallurgy, October, vol. 104, no. 9, 2004. pp. 517-527. [ Links ]
3. RIEKKOLA-VANHANEN, M. Finnish expert report on best available techniques in ferrochromium production. The Finnish Environment 314. Helsinki, Finnish Environment Institute, 1999. [ Links ]
4. ICDA (International Chromium Development Association), Statistical Bulletin, 2008 edition. 2008. [ Links ]
5. JONES, R. Pyrometallurgy in Southern Africa. 2011. http://www.pyromet-allurgy.co.za/PuroSA/index.htm (accessed 1 August 2011). [ Links ]
6. BEUKES, J.P., Dawson, N.F., and van Zyl, P.G. Theoretical and practical aspects of Cr(VI) in the South African FeCr industry, Journal of The Southern African Institute of Mining and Metallurgy, vol. 110, 2010. pp. 743-750. [ Links ]
7. PROCTOR, D.M., OTANI, J.M., FINLEY, B.L., PAUSTENBACH, D.J., BLAND, J.A., SPEIZER, N., AND SARGENT, E.V. Is hexavalent chromium carcinogenic via ingestion? A weight-of-evidence review. Journal of Toxicology and Environmental Health, Part A, vol. 65, 2002. pp.701-746. [ Links ]
8. IARC (International Agency for Research on Cancer), World Health Organization. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 49, Chromium, Nickel and Welding, 1997. [ Links ]
9. MA, G. AND GARBERS-CRAIG, A.M. A review on the characteristics, formation mechanisms and treatment processes of Cr(VI)-containing pyrometal-lurgical wastes. Journal of the Southern African Institute of Mining and Metallurgy, vol. 106, 2006. pp. 753-763. [ Links ]
10. MINTEK. Mintek Bulletin, 1993, no. 65, September 1993. [ Links ]
11. MINTEK. Mintek Bulletin, 1994, no. 71, March 1994. [ Links ]
12. Mintek. Mintek Bulletin, 1996, no. 95, May 1996. [ Links ]
13. SHEN, H. and FROSSBERG, E. An overview of recovery of metals from slag. Waste Management, vol. 23, 2003. pp. 939-949. [ Links ]
14. MASHANYARE, H.P. and GUEST, R.N. The recovery of ferrochrome from slag at Zimasco. Minerals Engineering, vol. 10, 1997. pp.1253-1258. [ Links ]
15. GIESEKKE, E.W. Mineral based treatment strategies for wastes and effluents. South African Journal of Science, vol. 95, 1999. pp. 367-372. [ Links ]
16. MA, G., and GARBERS-CRAIG, A.M. Stabilization of Cr(VI) in stainless steel plant dust through sintering using silica-rich. Journal of Hazardous Materials, vol. 169, 2009. pp. 210-216. [ Links ]
17. MAINE, C.F., SMIT, J.P., and GIESEKKE, E.W. The solid stabilization of soluble wastes generated in the South African ferrochrome industry, Final report to the Water Research Commission, 2005. WRCReport no 942/1/05. [ Links ]
18. GERICKE, W.A. Environmental aspects of ferrochrome production. Proceedings 7th International Ferroalloys Congress (INFACON XII), Trondheim, Norway, 1995. pp.131-140. [ Links ]
19. DOBSON, R.S. and BURGESS, J.E. Biological treatment of precious metal refinery wastewater: A review. Minerals Engineering, vol.20, 2007. pp.519-532. [ Links ]
20. MCBRIDE, C. Rainfall data for Burgersfort, Lydenburg, Brits and Rustenburg. Personal communication. 2009 SA Weather Service. [ Links ]
21. NIEMELA, P., KROGERUS, H., and OIKARINEN, P. Formation, characterization and utilization of CO-gas formed in ferrochrome smelting. Proceedings 10th International Ferroalloys Congress (INFACON X), Cape Town, South Africa, 2004. pp. 68-77. [ Links ]
22. MARCH, J. Advanced Organic Chemistry, 4th edn. John Wiley and Sons, USA.,1992. [ Links ]
23. FENDORF, S.E. and ZASOSKI, R.J. Chromium(III) oxidation by 8-MnO2. 1. Characterization. Environmental Science and Technology, vol. 26, 1992. pp. 79-85. [ Links ]
24. BARTLETT, R.J. Chromium cycling in soils and water: Links, gaps and methods. Environmental Health Perspectives, vol. 92, 1991. pp. 17-24. [ Links ]
25. EARY, L.E. and RAI, R. Kinetics of chromium(III) oxidation to chromium(VI) by reaction with manganese dioxide. Environmental Science and Technology, vol. 21, 1987. pp. 1187-1193. [ Links ]
26. WAZNE, M., MOON, D.H., JAGUPILLA, S.C., JAGUPILLA, S.C., CHRISTODOULATOS, C., DERMATAS, D., and CHRYSOCHOOU, M. Remediation of chromite ore processing residue using ferrous sulfate and calcium polysulfide. Geosciences Journal, vol. 11, 2007. pp. 105-110. [ Links ]
27. SU, C. and LUDWIG, R.D. Treatment of hexavalent chromium in chromite ore processing solid waste using a mixed reductant solution of ferrous sulphate and sodium dithionite. Environmental Science and Technology, vol. 39, 2005. pp. 6208-6216. [ Links ]
28. QIN, G., MCGUIRE, M.J., BLUTE, N.K., SEIDEL, C., and LEIGHTON, F. Hexavalent chromium removal by reduction with ferrous sulphate, coagulation, and filtration: A pilot-scale study. Environmental Science and Technology, vol. 39, 2005. pp. 6321-6327. [ Links ]
29. HE, Y.T., CHEN, C-C., and TRAINA, S.J. Inhibited Cr(VI) reduction by aqueous Fe(II) under hyperalkaline conditions. Environmental Science and Technology, vol. 38, 2004. pp. 5535-5539. [ Links ]
30. HWANG, I., BATCHELOR, B., SCHLAUTMAN, M.A., and WANG, R. Effects of ferrous iron and molecular oxygen on chromium(VI) redox kinetics in the presence of aquifer solids. Journal of Hazardous Materials, vol. B92, 2002. pp. 143-159. [ Links ]
31. SCHLAUTMAN, M.A. and HAN, I. Effect of Ph and dissolved oxygen on the reduction of hexavalent chromium by dissolved ferrous iron in poorly buffered aqueous systems. Water Research, vol.35, 2001. pp. 1534-1546. [ Links ]
32. BUERGE, I.J. and HUG, S.J. Kinetics and pH dependence of chromium(VI) reduction by iron(II). Environmental Science and Technology, vol. 31, 1997. pp. 1426-1432. [ Links ]
33. SEDLAK, D.L. and CHAN, P.G. Reduction of hexavalent chromium by ferrous iron. Geochimica et Cosmochimica Acta, vol. 61, 1997. pp. 2185-2192. [ Links ]
34. FENDORF, S.E. and LI, G. Kinetics of chromate reduction by ferrous iron. Environmental Science and Technology, vol. 30, 1996. pp. 1614-1617. [ Links ]
35. BEUKES, J.P., PIENAAR, J.J., LACHMANN, G., and GIESEKKE, E.W. The reduction of hexavalent chromium by sulphite in wastewater. Water SA, vol. 25, 1999. pp. 363-370. [ Links ]
36. BEUKES, J.P., PIENAAR, J.J., and LACHMANN, G. The reduction of hexavalent chromium by sulphate in wastewater - An explanation of the observed reactivity pattern. Water SA, vol. 26, 2000. pp. 393-395. [ Links ]
37. MUKHOPADHYAY, B., SUNDQUIST, J., and SCHMITZ, R.J. Removal of Cr(VI) from Cr-contaminated groundwater through electrochemical addition of Fe(II). Journal of Environmental Management, vol. 82, 2007. pp. 66-76. [ Links ]
38. LAKSHMIPATHIRAJ, P., RAJU, G.B., BASARIYA, M.R., PARVATHY, S., and PRABHAKAR, S. Removal of Cr(VI) by electrochemical reduction. Separation and Purification Technology, vol. 60, 2008. pp. 96-102. [ Links ]
39. TIAN, Y. and YANG, F. Reduction of hexavalent chromium by polypyrrole-modfied steel mesh electrode. Journal of Cleaner Production, vol. 15, 2007. pp. 1415-1418. [ Links ]
40. MARTINEZ, S.A. and RODRIQUES, M.G. Dynamic modeling of the electrochemical process to remove Cr(VI) from wastewaters in a tubular reactor. Journal of Chemical Technology and Biotechnology, vol. 82, 2007. pp. 582-587. [ Links ]
41. RUOTOLO, L.A.M., SANTOS-JUNIOR, D.S., and GUBULIN, J.C. Electrochemical treatment of effluents containing Cr(VI). Influence of pH and current on the kinetics. Water Research, vol. 40, 2006. pp. 1555-1560. [ Links ]
42. RODRIGUEZ, M.G. and MARTINEZ, S.A. Removal of Cr(VI) from wastewaters in a tubular electrochemical reactor. Journal of Environmental Science and Health Part A, vol. 40, 2005. pp. 2215-2225. [ Links ]
43. RODRIGUEZ-VALADEZ, F., ORTIZ-EXIGA, C., IBANEZ, J.G., ALATORRE-ORDAZ, A., and GUTIERREZ-GRANADOS, S. Electroreduction of Cr(VI) to Cr(III) on reticulated vitreous carbon electrodes in a parallel-plate reactor with recirculation. Environmental Science and Technology, vol. 39, 2005. pp. 1875-1879. [ Links ]
44. RUOTOLO, L.A.M. and GUBULIN, J.C. A factorial-design study of the variables affecting the electrochemical reduction of Cr(VI) at polyaniline-modified electrodes. Chemical Engineering Journal, vol. 110, 2005. pp. 113-121. [ Links ]
45. HUNSOM, M., PRUKSATHORN, K., DAMRONGLERD, S., VERGNES, H., and DUVERNEUIL, P. Electrochemical treatment of heavy metals (Cu2+, Cr6+, Ni2+) from industrial effluent and modelling of copper reduction. Water Research, vol. 39, 2005. pp. 610-616. [ Links ]
46. AGUILAR, R., MARTINEZ, S.A., RODRIGUEZ, M.G., and SOTO, G. Process analysis for treatment of industrial plating wastewater: simulation and control approach. Chemical Engineering Journal, vol. 105, 2005. pp. 139-145. [ Links ]
47. GUZMAN-PANTOJA, J., IBANEZ, J.G., VASQUEZ-MEDRANO, R.C., and OROPEZA - GUZMAN, M.T. Direct electrochemical reduction of hexavalent chromium in a filter-press reactor. Bulletin of Electrochemistry, vol. 20, 2004. pp. 107-114. [ Links ]
48. BARRERA-DIAZ, C., PALOMAR-PARDAVE, M., ROMERO-ROMO, M., and MARTINEZ, S. Chemical and electrochemical considerations on the removal process of hexavalent chromium from aqueous media. Journal of Applied Electrochemistry, vol. 33, 2003. pp. 61-71. [ Links ]
49. CHAUDHARY, A.J., GOSWAMI, N.C., and GRIMES, S.M. Electrolytic removal of hexavalent chromium from aqueous solutions. Journal of Chemical Technology and Biotechnology, vol. 78, 2003. pp. 877-883. [ Links ]
50. VILAR, E.O., CAVALCANTI, E.B., CARVALHO, H.R., and SOUSA, F.B. Cr(VI) electrochemical reduction using RVG 4000 graphite felt as the electrode. Brazilian Journal of Chemical Engineering, vol. 20, 2003. pp. 291-303. [ Links ]
51. EL-SHOUBARY, Y., SPEIZER, N, SETH, S., and SAVOIA, H. Pilot plant to treat chromium-contaminated groundwater. Environmental Progress, vol. 17, 1998. pp. 209-213. [ Links ]
52. ABDO, M.S.E. and SEDAHMED, G.G. A new technique for removing hexavalent chromium from waste water and energy generation via galvanic reduction with scrap iron. Energy Conversion and Management, vol. 39, 1998. pp. 943-951. [ Links ]
53. VLYSSIDES, A.G. and ISRAILIDES, C.J. Detoxification of tannery waste liquors with an electrolysis system. Environmental Pollution, vol. 97, 1997. pp. 147-152. [ Links ]
54. SANDERS, C.C. Electrochemical reduction of Cr(VI) in the decontamination process ELDECON. Waste Management, vol. 16, 1996. pp. 683-689. [ Links ]
55. ECODOSE. 2011. http://www.ecodose.co.za/about_us.html (accessed 4 August 2011) [ Links ]
56. GERICKE, W.A. Bacterial reduction of hexavalent Cr: A viable environmental solution to the treatment of effluent from A FeCr smelter. Proceedings 9th International Ferroalloys Congress (INFACON IX), Quebec City, Canada, 2001. pp. 438-443. [ Links ]
Paper received Aug. 2011
Revised paper received Jan. 2012














