Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 no.4 Johannesburg abr. 2012
JOURNAL PAPER
Cyanidation of reef and surface gold ores
L. Rademan; D.R. Groot
Department of Materials Science and Metallurgical Engineering, University of Pretoria
SYNOPSIS
The kinetic leaching behaviour of a low-grade surface gold ore and a high-grade reef ore were evaluated to determine the achievable gold recovery from these ores and particularly mixtures of these ores. The reef ore material has a head grade of 13.40 g/t with a relative standard deviation of 3%, and the unprocessed surface ore material a head grade of 0.43 g/t with a relative standard deviation of 15.6%. It is concluded that the kinetic leaching behaviour of these free milling gold ores during cyanidation, is not influenced by mixing of reef and surface ore material. No statistically significant relationship exists between the achievable recovery by direct cyanidation and the head grade of the material fed. No ideal ratio of surface ore to reef ore could be established. It is recommended that these materials should be processed simultaneously to minimize the costs, security risks, and losses incurred in transportation.
Keywords: leaching kinetics, gold, types of gold ores, cyanidation, Witwatersrand gold ores
Introduction
Currently, at Gold Fields Ltd Kloof division a surface ore with typical head grade of 0.72 g/t is processed separately from a reef ore with a typical head grade of 6.84 g/t. The separate processing is considered an industrial problem as it results in excessive transportation costs, increased security risks, and losses due to the double handling of the material. The reef ore is hoisted at various shafts and processed at Kloof No. 2 plant as shown in Figure 1, with the implication of the transportation routes indicated. The surface ore is unprocessed stockpiled material that is processed at Kloof No. 1 Plant.
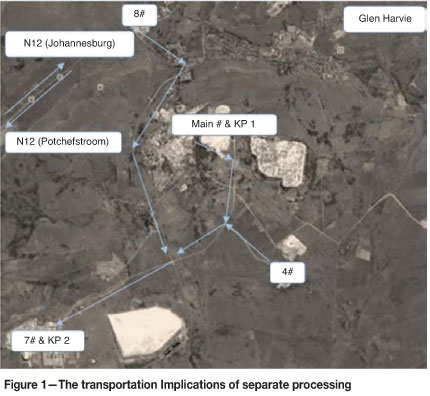
The separate processing of these ores is a result of the kinetic leaching behaviour of these ores, and particularly mixtures of these ores, not being properly understood. It is believed that simultaneous processing of these ores results in decreased gold recoveries. However, if the kinetic leaching behaviour of these free-milling Witwatersrand gold ores can be properly understood, the separate processing can possibly be minimized and even eliminated. Consequently, the primary aim of the investigation was to determine the kinetic leaching behaviour of these gold ores and particularly of mixtures of these ores.
Gold resources
South Africa has produced more than a third of the total world gold output and has been a top gold producer for many years. However, in more recent years there has been a decrease in production due to deeper mining for lower grades of gold. The gold ores that were evaluated are from the Witwatersrand Basin, which is the largest gold deposit in South Africa and has been mined for over 120 years1.
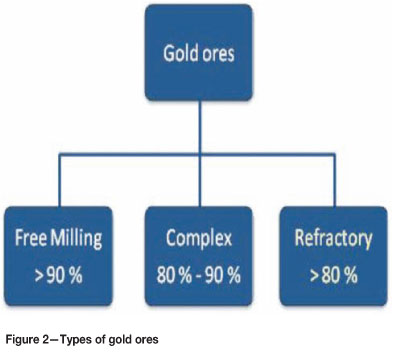
Gold ores can be classified according to the gold recovery achievable by direct cyanidation as free-milling, complex, or refractory as shown in Figure 2. Gold from free-milling gold can easily be recovered by direct cyanidation, whereas complex gold ores reagent consumption tends to increase due to higher copper and iron contents. Refractory gold ores require extensive pre-treatment prior to cyanidation for economical achievable recoveries2.
Experimental approach
Sampling
Approximately 80 kg of each ore was sampled from the respective conveyor at Kloof No. 1 plant. The samples were collected at four hourly intervals. The reef ore is hoisted from the shaft at a particle size of + 150 mm and was crushed by a mini-jaw crusher on site to -100 mm. In order to prevent contamination of the reef ore sample, the mini-jaw crusher was washed and subsequently flushed with 5 kg of the collected sample. The surface ore enters the plant at a particle size -150 mm and can be easily handled. Both the reef ore sample and surface ore sample were dried and pulverized to 80 per cent passing 75 ìm, which is the optimal particle size for cyanidation. The samples were homogenized by a rotary splitter into 300 g units from which the various feed mixtures were prepared.
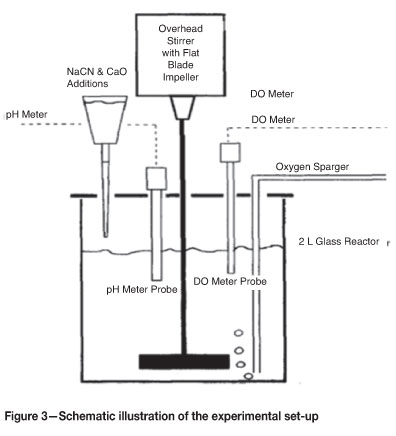
Experimental set-up
A series of cyanidation tests with various feed mixtures in multiples of 25 per cent was conducted in a 2 l glass reactor as shown in Figure 3. The reactor was fitted with an overhead stirrer run at a rotational speed of 600 r/min, and was closed with a lid that provided ports for the oxygen sparger, the pH meter probe, and the dissolved oxygen probe.
Cyanide concentrations were determined by means of titration with silver nitrate throughout each experiment. The oxygen levels were maintained above 6.2 ppm and monitored throughout each experiment. The leaching parameters used are shown in Table I.
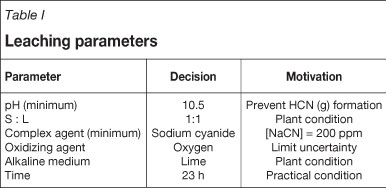
During preliminary test work, the sodium cyanide concentration was determined as 200 ppm. By maintaining the cyanide concentrations above 200 ppm it was possible to remain above the proposed level of 160 ppm even after the 23-hour leach duration. Oxygen was used as opposed to air primarily in order to selectively observe the effect of various feed mixtures on the kinetic leaching behaviour, as well as to improve the stability of the conditions during the experiment. The use of air increases reagent consumption and consequently causes instability in the experimental conditions. The leaching time of 23 hours was chosen to allow for safe work within recommended working hours.
Procedure
A slurry was prepared from 1 kg of feed mixture and 1 l distilled water and subjected to a 1 hour pre-conditioning stage, during which the pH was adjusted to 11 and oxygen sparged into the slurry. This improves oxygen levels during the experiments as well as minimizing the reagent consumption. After the one hour preconditioning step an initial sodium cyanide mass of 0.4 g was added and the experiment was commenced. Kinetic sampling was conducted at 30 minutes, 1 hour, 2 hours, 4 hours, 8 hours, and finally at 23 hours. A volume of 120 ml was withdrawn and immediately filtered. The gold concentrations in the solutions were analysed by atomic absorption spectrometry (AAS). The final residue was washed and repulped prior to analysis such to improve the recoveries. Both the accumulated residues from kinetic sampling as well as the final residues from each experiment were evaluated for the amount of gold by fire assaying at Driefontein Laboratory. Each experiment was duplicated for verification.
Results and discussion
The expected low kinetic leaching behaviour of the surface ore material and various mixtures thereof can be due only to varying compositional effects within the two ore compositions.
Plant personnel were of the opinion that mixing the reef and surface ore material led to poor gold recoveries. This is possibly a result of slower leaching, which could be due to different compositions of the ores. This would result in increased reagent consumption by the surface ore material, leading to decreased kinetic behaviour due to competing reactions as well as insufficient reagent being available for the leaching of the gold particles. Consequently, the compositional effects, achievable gold recovery, and leaching kinetic behaviour were evaluated and compared for the two ores.
Mineral composition
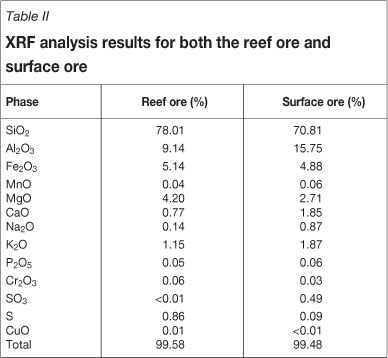
The mineral composition of both the reef and surface ores was determined by X-ray fluorescence (XRF) analysis to determine whether any compositional variations are present which could influence the kinetic leaching behaviour. The results in Table II clearly indicate that these ores contain very low sulphide levels present and are thus oxide gold ores. However, the severe effect of the presence of the sulphides on reagent consumption must not be ignored, even at these low levels. The two ores have very similar compositions with some significant levels of iron and copper relative to that of the gold.
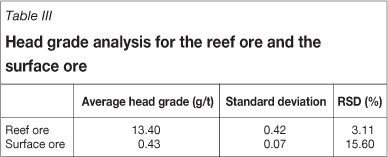
The head grade for the reef ore and surface ore was determined by fire assay, with the results indicated in Table III for five repeats. The reef ore material had an average head grade of 13.40 g/t with a relative standard deviation of 3.11 per cent, which is acceptable as it remains within a reasonable error margin below 5 per cent. However, the surface ore had an average head grade of 0.43 g/t with a relative standard deviation of 15.60 per cent. This grade is lower than that which was expected for this stockpiled material. The large relative standard deviation determined for the stockpiled material is a result of the difficulty in obtaining a representative sample from such a stockpile, which has been created from a wide variety of material grades over many years. The large relative standard deviation for the surface ore holds implications for the interpretation of the results found, as a large error margin is induced.
Mass balance
A summary of the mass balance for each experiment is shown in Table IV which indicates the mass of gold in the feed, the gold present in both the accumulated residues from the kinetic sampling, as well as the final residues, the losses to the solutions of the kinetic sampling and finally the gold present in the final solutions. The gold extraction with reference to the solids analysis was very good compared to that which was expected. These recoveries clearly indicate that the two samples are indeed free-milling gold ores. This is an important result as it indicates that it is highly unlikely for the surface ore material to cause a decreased gold recovery rate when blended with the reef ore material. The gold extractions based on the solution analyses show larger discrepancies to that which was expected and are not as reliable.
However, the achievable gold recoveries remain within reasonable limits at the 95% confidence interval (ì ±2ó) for the identified relative standard deviation (RSD) of the feed materials. The discrepancies that are found are possibly a result of unavoidable deterioration of the kinetic samples prior to analysis. Severe deterioration of the reef material kinetic solution samples was observed with time. The 95 per cent confidence intervals as based on solution analyses for the various feed mixtures are shown in Table V.
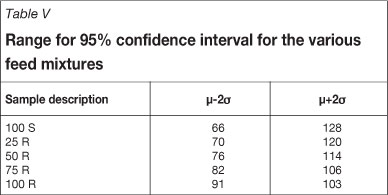
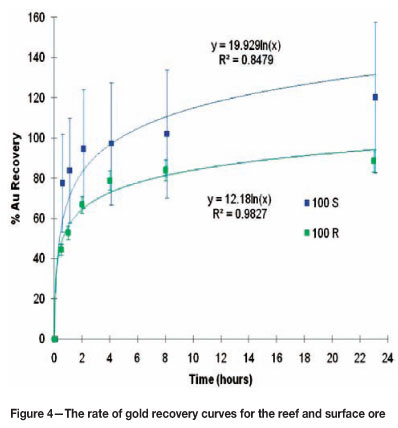
Figure 4 shows the rate of gold recovery curve for the 100 per cent reef ore sample and the 100 per cent surface ore. The gold recovery curves for the various mixtures follow the typical shape as expected from literature. It is clear from Figure 4 that the 100 per cent reef ore sample has a higher initial leaching rate than was expected. The reason for this, however, is uncertain. It is important to note that the curves are based on solution analyses, and as seen in Table IV the results based on the solution analyses are less reliable than those based on the solids. Although the curves show a relatively high variance between the two ores, it is expected that if solid analyses were used that these curves would be closer to one another.
The rate of gold recovery curves for the various mixtures were found to remain in between that of the 100 per cent reef and 100 per cent surface ore material, and are shown in Figure 5. The initial rate of leaching is again typically large and decreases as time progresses similarly to the 100 per cent reef and 100 per cent surface ore material.
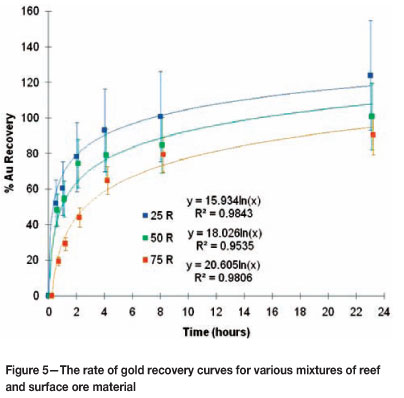
From inspection of the achievable gold recoveries, shown in both the mass balances and the recovery curves, it can be deduced that the achievable gold recovery is independent of the material fed to the leaching test, irrespective of the ratio of reef to surface material. Again, it is important to note that the results as based on the solid analyses are situated very close to one another. Consequently, the kinetic leaching behaviour is not influenced by the ratio of material fed and no ideal ratio of reef to surface ore mixture can be identified at this stage under the conditions of this test work. Other factors that may influence the kinetic leaching behaviour during cyanidation could be the large quantities of both iron and copper, which are recovered in greater quantities than gold for all mixtures of reef and surface ore material fed to cyanidation tests.
Leaching kinetics
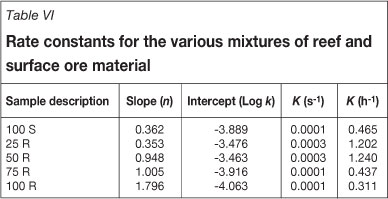
The data was also evaluated in order to determine whether the first- order rate equation applies for the cyanidation tests that were conducted. A log-log plot of the gold concentration against the leaching rate revealed a straight line for each test with a slope (n) and intercept (log K) as seen in Table VI. The rate constant, K, for the various mixtures shows a very good relationship to that provided in the literature. Typical rate constants for Witwatersrand ores3 in the range of 0.4 h-1 to 1.0 h-1. It is, however, clear that the slope, n, or the order of the reaction does not indicate first- order kinetics.
In order to validate the above estimate for the order of the kinetic reaction, the integral method was used to evaluate the data. The integral method enables the order of the reaction to be determined dependent on the best fit of a straight line through the data points of the batch reactor leaching test4. Figures 6-8 show the straight line fit to the data for the mixture containing 50 per cent reef ore and 50 per cent surface ore.
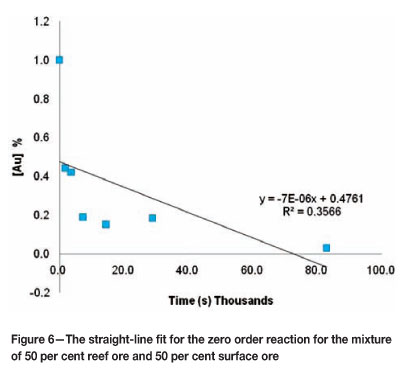
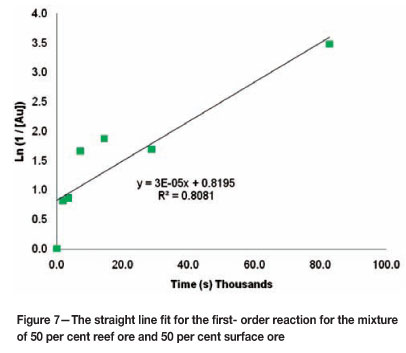
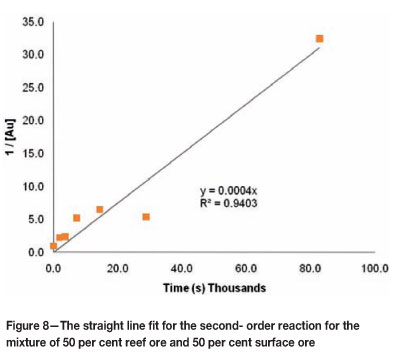
These figures indicate that for the mixture of 50 per cent reef ore and 50 per cent surface ore, the data are best represented by a second- order kinetic reaction. Similar tests were done for all the mixtures used and the results are shown in Table VII.
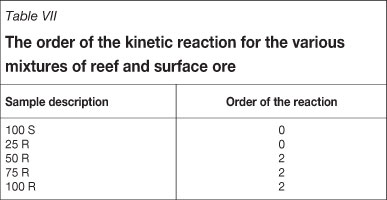
There is reasonable agreement between the two approaches. Unfortunately, the analyses are based on the solution results, which are not as reliable as would be desired.
Conclusion
It can be concluded from the results shown that both these ores are indeed free-milling gold ores. Furthermore, that the kinetic leaching behaviour during cyanidation of these
Witwatersrand ores is not influenced by the various mixtures of reef and surface ore material, under the conditions of this test work. No relationship was found between the head grade of the feed material and the achievable recovery by cyanidation within the experimental uncertainties indicated. No ideal ratio could be established from the experimental data. Another effect that could influence the kinetic leaching behaviour during cyanidation for these free-milling gold ores is the significant presence of the reagent consumers iron and copper. It is recommended that simultaneous processing of the reef and surface ore material should be undertaken the transportation costs, security risks, and losses due to transportation can be minimized and even eliminated. The metallurgical accounting of the two plants will also benefit, as a decrease in undetermined material losses will result.
Acknowledgements
The author would like to thank both Gold Fields Ltd Kloof division and the University of Pretoria for their guidance and assistance throughout the project.
References
1. HANDLEY, J. World gold resources: production rises marginally while consumption is still in excess- Stocks at surface remain the big market factor. Economic Geology Research Unit, University of the Witwatersrand, Johannesburg, 1997. [ Links ]
2. LA BROOY, S.R., LINGE, H.G., and WALKER, G.S. Review of gold extraction from ores. Minerals Engineering, vol. 7, no. 10, 1994. pp. 1213-1241. [ Links ]
3. NICOL, M.J., FLEMING, C.A., and CROMBERGE, G. The adsorption of gold onto activated carbon, Journal of the South African Institute of Mining and Metallurgy, vol. 84, no. 3, 1984. pp. 70-78. [ Links ]
4. FOGLER, H.S. Elements of Chemical Reaction engineering. 2nd edn.. Prentice Hall. 1992. pp. 200-205. [ Links ]
Paper received Feb. 2012
Revised paper received Feb. 2011
Paper written on project work carried out in partial fulfilment of B. Eng (Metallurgical Engineering)














