Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 no.4 Johannesburg Abr. 2012
JOURNAL PAPERS
Precipitation of rhodium from a copper sulphate leach solution in the selenium/tellurium removal section of a base metal refinery
C. LotteringI; J.J. EksteenII; N. SteenekamptII
IDepartment of Process Engineering, Stellenbosch University, South Africa
IILonmin, Western Platinum, Process Division, Marikana, South Africa
SYNOPSIS
Copper sulphate solutions are produced during the pressure leaching of first-stage leach residue in a typical base metal refinery process. Apart from impurities such as selenium and tellurium, this leach solution also contains other precious metals (Rh, Ru, and Ir) due to dissolution in the pressure leaching stage. Selenium and tellurium are removed from the leach solution by precipitation with sulphurous acid, since these elements negatively affect electrowinning. This study investigates the feasibility of modifying the Se/Te precipitation process step to achieve significant rhodium precipitation in addition to selenium and tellurium precipitation, in order to reduce the rhodium inventory of the process.
Thiourea, SO2, formaldehyde, formic acid, and sodium thiosulphate were evaluated to determine which of these reagents would achieve the highest Rh precipitation. Based on these screening tests, SO2 and thiourea were selected to be used as precipitation reagents in optimization tests. During these optimization tests, the operating temperature (80 to 150°C) and the amount of reagent added (80 to 140 per cent excess) were varied to evaluate the effects that these operating conditions have on the precipitation behaviour of Rh as well as Se, Te, Cu, and Ni, and to propose appropriate operating conditions.
It was found that thiourea resulted in good Rh and Se precipitation (typically in excess of 90 per cent), but precipitated noticeably more copper and nickel from the solution than when SO2 was used. In addition, thiourea had poor Te removal characteristics. SO2 achieved a maximum of 70 per cent precipitation of the rhodium. Faster kinetics and a larger extent of Te precipitation were, however, observed when using SO2 compared to thiourea.
Keywords: rhodium, copper, selenium, tellurium, iron, base metal, thiourea, sulphur dioxide, precipitation, ionic, co-precipitation, cationic substitution, cementation.
Introduction
Platinum group metals (PGMs) typically occur in ore deposits containing noticeable amounts of base metals. As a result, the concentration and refining process usually includes a base metal refinery (BMR) where the base metals and PGMs are separated via a number of pyrometallurgical and hydrometallurgical processing steps. More specifically, the hydrometallurgical process route often entails various stages of leaching to dissolve primarily base metals from the converter matte. It has, however, been noted that a significant amount of the other precious metals (OPMs, which include Rh, Ru, and Ir) also dissolves in these leaching stages, yielding a pregnant base metal leach solution containing small quantities of OPMs (Dorfling et al., 2011). In addition to the OPMs, the pregnant leach solution also contains trace amounts of impurities such as selenium and tellurium. Before final processing of the leach solution can take place to produce the base metals, selenium and tellurium must be removed to prevent undesirable effects in downstream processes, particularly electrowinning. Currently, Se and Te are removed by means of precipitation with sulphurous acid.After selenium/tellurium removal, the solution is sent for further processing in order to recover base metals. A large OPM inventory (and potentially OPM losses) results if the leach solution from which the base metals have been removed is recycled to the leaching stages. It is thus in the interest of companies producing PGMs to investigate methods to remove OPMs from the pregnant base metal leach solution prior to base metal recovery.
It has been observed that approximately 20 per cent of the rhodium in solution is unintentionally precipitated in the Se/Te precipitation stage. This method was therefore examined as a possible means of removing larger amounts (in excess of 90 per cent) of the rhodium from solution by adjusting the operating conditions to obtain optimum rhodium precipitation. Previous research by McGeorge et al. (2009) suggested that Rh precipitation would be possible under the correct operating conditions and with the use of an appropriate reagent.
The main objectives of the project were to determine which reagent would yield the best rhodium precipitation from the copper sulphate leach solution, and to determine the effect that temperature and the amount of reagent addition would have on the precipitation behaviour, in order to propose operating conditions that would result in the desired precipitation efficiencies.
Literature review
General base metal refinery process
Figure 1 shows the basic process used for the production of base metals and PGMs in a typical base metal refinery. Matte from the smelting section of the plant is milled before being sent to the atmospheric leaching stage, where sulphuric acid is used to dissolve most of the nickel in the matte. The pregnant leach solution leaving this leaching stage is sent to nickel crystallizers, where nickel sulphate crystals are produced.
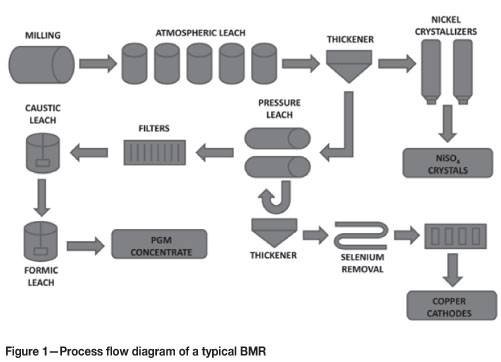
The solids remaining after the atmospheric leaching stage are sent to the pressure leaching stage, where most of the copper in the first-stage leach residue is dissolved together with impurities such as Te and Se, as well as small quantities of OPMs. The solid residue leaving the pressure leaching stage contains a high concentration of PGMs, and is treated in batch leaching processes and the platinum metal refinery in order to produce high-purity PGMs.
The pregnant leach solution recovered from the pressure leaching stage is sent to the selenium/tellurium removal section, where these species are removed by precipitation with sulphurous acid. This processing step is important in order to avoid problems that may occur in copper electrowinning as a result of the presence of these impurities (Weir et al., 1980). It is also during this Se/Te precipitation step that partial rhodium precipitation takes place unexpectedly. Once Se and Te have been removed, the pregnant leach solution is sent to electrowinning for copper recovery.
Se/Te removal process
As discussed, the removal of selenium and tellurium from the copper sulphate leach solution is typically achieved by precipitation with sulphurous acid. The precipitation reactions in the Se/Te removal section would thus be as shown in reaction [1] and reaction [2] (Sherritt Gordon Mines Ltd, 1983):
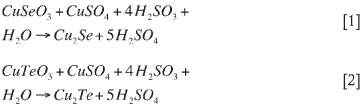
Selenium removal and the extent to which precipitation occurs is dependent on the presence of the different Se ions, since Se6+ ions are typically more difficult to remove than Se4+ ions due to their low reactivity (Hofirek, 1983).
Unlike selenium, it is unlikely that tellurium will be directly reduced by sulphur dioxide in a sulphuric acid matrix, when copper is present in the solution (Shibasaki et al., 1991). For Te to precipitate from solution, it has been suggested that it would be more likely that the SO2 would reduce copper from cupric to cuprous sulphate, resulting in the formation of elemental copper. Tellurium would then precipitate by secondary cementation onto the surface of the elemental copper particles (Wang et al., 2003). These reactions are shown for tetravalent- and hexavalent Te in reactions [3] and [4]:
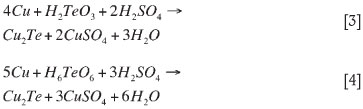
Effect of iron
In addition to Se and Te, the other major impurity that generally occurs in the solutions is iron, in concentrations up to 1.6 g/l. The presence of iron in the solution has to be taken into account since it reacts with the precipitation reagent, consuming a large portion of the reagent added. In order to determine the amount of reagent to be added, it is thus necessary to take the reaction of iron with each reagent into account.
Since the concentration of iron in solution is an order of magnitude larger than that of selenium and tellurium, all percentage excess values used in the experimental calculations for the various reagents were calculated based on the amount of iron in the solution. The reaction of iron with each of the reagents investigated as part of this project is shown in reactions [5] to [12].
Sherritt Gordon Mines Ltd (1983) suggested that the reaction of iron with SO2 is as follows:
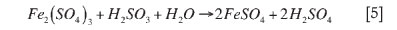
The reaction of thiourea with the iron sulphate was determined from literature (Mensah-Biney et al., 1994):
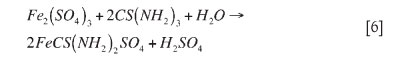
McGeorge et al. (2009) formulated the reaction of the iron compound with an aqueous thiosulphate reagent:
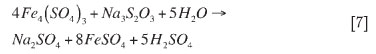
No information was available in the literature for the reactions of formaldehyde and formic acid with iron. Therefore, half reactions were formulated for each of these reagents and these were used to determine a suggested overall reaction with each reagent. Habashi (2009) suggests that precipitation involving formic acid takes place via ionic precipitation. The overall reaction suggested for this would then be:
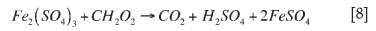
Formaldehyde, on the other hand, is known to form precipitates via non-ionic precipitation. For this reason, there are two possible ways in which formaldehyde could react. Formaldehyde could react directly with the iron, resulting in the formation of formic acid. It could then be assumed that the reaction has achieved completion and goes no further. Alternatively, the formic acid could continue to react with the iron. Ultimately, both of these reactions would result in an overall reaction as shown in reaction [9].
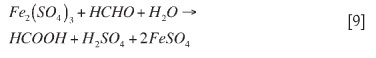
Rhodium precipitation theory
The mechanism of rhodium precipitation from copper sulphate solutions was studied by McGeorge et al. (2009), who used sodium thiosulphate as the precipitation reagent. From this study, it was suggested that rhodium precipitation takes place via homogeneous reactions initially. Here, the copper and rhodium present in the solution precipitate by ionic precipitation, as shown in reactions [10] and [11] (McGeorge et al., 2009):
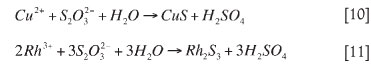
If rhodium was present in the solution without copper, it would precipitate according to reaction [11]. Since the concentration of copper in the solution is approximately three times greater than that of rhodium, copper consumes more of the reagent than rhodium, and precipitates faster. These reactions occur before noticeable nucleation, due to an induction period created by the thiosulphate forming complexes. Copper then continues to precipitate via heterogeneous growth, as shown in reaction [12] (McGeorge et al.,2009):
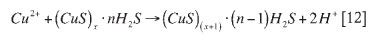
Once the copper concentration has dropped, rhodium can continue to precipitate by co-precipitation or by cationic substitution. Co-precipitation involves rhodium and copper precipitating together, as shown in reactions [13] and [14] (McGeorge et al., 2009):
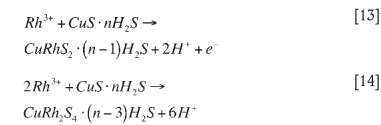
When cationic substitution takes place, the rhodium substitutes for the copper in the copper sulphide formed during the homogeneous reaction phase. This process occurs as shown in reaction [15] (McGeorge et al., 2009):
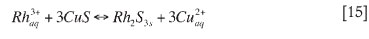
Cationic substitution is the slowest method by which rhodium can precipitate, while ionic precipitation is the fastest (due to the large excess in sulphur fed).
Experimental
Experimental plan
The experimental work was divided into two sections. The first group of experiments comprised the screening tests, which were performed to allow for the selection of reagents that would be used in further tests (optimization tests). Optimization tests (the second group of tests) were performed to determine the applicable operating temperature and reagent addition amount for the selected reagents. All the tests were done using process solution supplied by Western Platinum Ltd., collected prior to the selenium/tellurium stage at their BMR.
Five different tests were performed as part of the screening tests. These tests were carried out at a temperature of 150°C for the five reagents that were identified as being potentially able to precipitate the rhodium. These reagents include sodium thiosulphate, sulphur dioxide, formaldehyde, formic acid, and thiourea. As mentioned previously, the amount of reagent to be added was calculated based on the amount of iron present in the solution. A percentage excess value was selected for each experiment and the volume of reagent required to achieve this excess then calculated.
Based on the results of the screening tests, it was decided that thiourea and SO2 would be used for the optimization tests. These tests were operated at the conditions summarized in Table I. The pressure in the autoclave was not explicitly controlled; all experiments were performed at the vapour pressure of solution at the experimental temperature of the particular run.
Experimental equipment
The experiments were carried out in a two-litre Büchiglasuster autoclave manufactured from Hastelloy. The autoclave was equipped with automatic temperature control, with heating by means of electric heating elements and cooling by control of a valve regulating the flow of cooling water through the autoclave jacket. The solution in the autoclave was agitated using an anchor stirrer driven by a magnetic stirrer drive, for which the agitation speed could be set at a fixed value. A 300 ml stainless steel cylinder with isolation valves connected to the autoclave cover plate allowed the addition of reagents under pressure. Nitrogen was used to pressurize the reagent cylinder, and was sparged into the solution by means of a tube extending from the cover plate into the solution. Sampling was done through the autoclave drain valve, which was located at the bottom of the autoclave.
Operating procedure
Pressure leach solution (700 ml) was placed into the autoclave, the autoclave was closed and all valves shut. The process solution was heated to the desired temperature while being agitated at 250 r/min. While the process solution was being heated, the correct quantity of reagent was placed into the reagent cylinder and the cylinder was pressurized using nitrogen. The cylinder was then attached to the cylinder attachment point on the autoclave cover plate.
Once the operating temperature had been reached, a sample was taken prior to opening the valve at the base of the reagent cylinder, which allowed the reagent to flow into the process solution.
After addition of the precipitation reagent, samples were taken every 20 minutes for the first hour, every 30 minutes for the second hour, and every two hours thereafter, for the full 8-hour duration of the experiment.
All samples were analysed for copper, nickel, selenium, tellurium, and rhodium using ICP analysis. Little precipitation of copper and nickel is desirable, since these elements are recovered in downstream processes following Se/Te removal. The precipitation of Se, Te, and Rh, on the other hand, should ideally be maximized.
Results and discussion
Screening tests
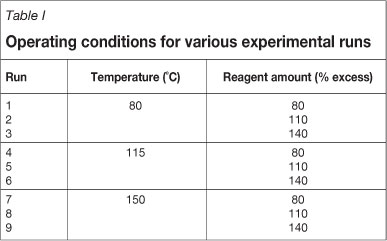
When analysing the results for the screening tests, it is important to note that the initial percentage excess was calculated to be 180 per cent for each reagent, based on the assumption that all reagents would react in a similar way to the SO2 with the iron. The stoichiometry of the iron-SO2 reaction was thus used as a basis. Once the process chemistry was better understood (as discussed earlier), the actual percentage excess of each reagent used was calculated. The results of these calculations are shown in Figure 2.
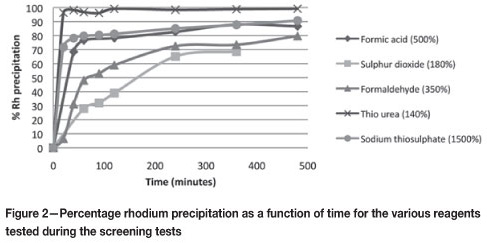
Figure 2 shows the percentage rhodium precipitation as a function of time for each of the screening tests run with the various reagents. The precipitation of rhodium was used as the only performance measurement to determine which of the reagents would be investigated further during the optimization tests. It was furthermore assumed that the amount of precipitation would increase with an increase in the amount of reagent added. This was based on the fact that increasing the degree of supersaturation increases precipitation (Söhnel and Garside, 1992).
Although the amount of thiourea used was the smallest in terms of percentage excess, this reagent provided the highest rhodium precipitation percentage. In addition, kinetics for this precipitation were fast, as evidenced by the steep precipitation curve seen in Figure 2 (approximately 97% of the rhodium had been precipitated 20 minutes after addition of the thiourea). For these reasons, thiourea was selected as one of the reagents for further tests. Sulphur dioxide was selected as the second reagent to be investigated during the optimization tests, since it is currently used as the reagent for Se/Te removal on site. Limited modifications to the current process would thus be required if this reagent were used for Rh precipitation.
Sodium thiosulphate was used to obtain data that could be compared with published data. This reagent would not be considered for practical implementation, since this would lead to the addition of sodium to the BMR process circuit. Sodium can be removed from the process only at the nickel crystal-lizers, and this would inevitably lead to a reduction in the quality of the nickel sulphate crystals. In general, it can be said that the results achieved with sodium thiosulphate during this study are comparable with those reported in the literature (McGeorge et al., 2009).
Optimization tests
Selenium and tellurium
Figure 3 shows the precipitation of Se achieved with the different reagents at different temperatures. It seems evident that Se precipitation greater than 90 per cent is achieved quickly with both thiourea and SO2, regardless of the temperature at which the experiment is run. In addition, the amount of reagent seems to have little effect on the extent or kinetics of precipitation. These observations are in agreement with the results obtained in the study performed by Harañczyk et al. (2002).
Weir et al. (1980) suggested that Se precipitation is better at lower temperatures, since approximately 99 per cent of the Se(IV) ions precipitate at all temperatures, but Se(VI) precipitation decreases from 90 per cent as the temperature increases above 50°C. This is possibly due to the higher solubility of the Se(VI) ions, making these more difficult to precipitate than the Se(IV) ions. Increasing the temperature increases the solubility limit of the solution and allows the more soluble ions to return to solution. This may explain why, for SO2, the amount of Se precipitated at 150°C is lower at any given time for the same percentage excess values. Thiourea has been found to act as a catalyst for Se precipitation, which may explain why the amount of Se precipitated with thiourea does not decrease at higher temperatures (Weir et al., 1982).
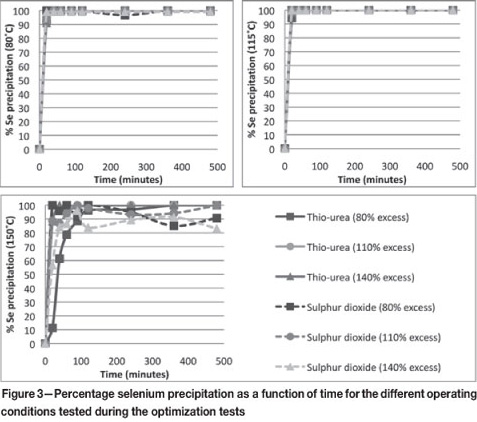
From Figure 4, it seems that thiourea has difficulty removing tellurium, especially at low temperatures. A possible explanation for this is the fact that tellurium precipitation takes place by secondary cementation on elemental copper. It may be that thiourea precipitates copper in a form that is not available for cementation, limiting the amount of tellurium that can precipitate. At higher temperatures, with more copper precipitating (refer to Figure 6), more copper may precipitate in a form suitable for cementation. This may be why tellurium precipitation with thiourea at higher temperatures is better than at lower temperatures.
At lower temperatures, there appears to exist an optimum residence time to achieve maximum tellurium precipitation with SO2. A possible explanation for this observation is the fact that precipitation is dependent on residence time (Söhnel and Garside, 1992). As residence time increases, more tellurium is precipitated from the solution, decreasing the amount of tellurium present in the solution and thus adjusting the supersaturation level of the solution (in a batch process). Eventually, the Te level in the solution falls so low that the tellurium precipitate starts to redissolve.
For the tellurium concentration in the solution to decrease to below its solubility limit, the tellurium precipitation rate must be significantly faster than the dissolution rate of the precipitate initially. As the amount of precipitate increases, the dissolution rate will increase. The dissolution rate must surpass the precipitation rate at some point for the percentage tellurium precipitation to decrease. The system equilibrium is eventually achieved when the net rate of dissolution is equal to zero.
The Te precipitation trend at higher temperatures (150°C and 140 per cent excess SO2 at 115°C) does not follow the same trend as at lower temperatures. This could indicate that the rate of dissolution of rhodium is more dependent on the temperature of the system than the rate of precipitation. Thus, when the temperature is higher, the dissolution rate increases more quickly, while the rate of precipitation remains relatively constant. Then, the system can achieve equilibrium faster.
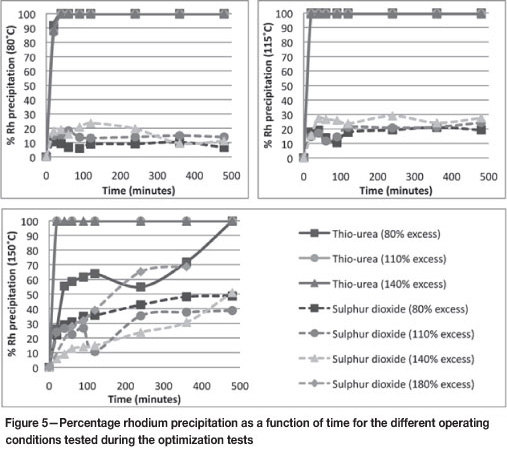
Rhodium
It seems from Figure 5 that thiourea precipitates almost 100 per cent of the rhodium from the solution at all temperatures, regardless of the amount of reagent added. In addition, the kinetics of this precipitation reaction appear to be fast under all operating conditions.
Figure 5 also shows the Rh precipitation achieved with SO2. Although this precipitation is significantly lower than that achieved with thiourea at lower temperatures, precipitation with this reagent seems to be heavily dependent on the operating conditions. A comparison of the rhodium precipitation achieved with SO2 for the same percentage excess at different temperatures shows that larger reagent quantities seem to lead to greater precipitation of rhodium.
Using SO2 at 150°C, the amount of rhodium precipitated increases as the percentage excess reagent increases, if the percentage precipitation at the end of the test is considered. When using 180 per cent excess SO2 at 150°C, overall rhodium precipitation of approximately 70 per cent can be achieved. It is thus evident that the optimum operating conditions for rhodium precipitation with SO2 have not been determined, but there are sufficient indications that percentage precipitation values in excess of 70 per cent can be achieved by increasing the percentage excess reagent and/or the operating temperature even further.
Copper and nickel
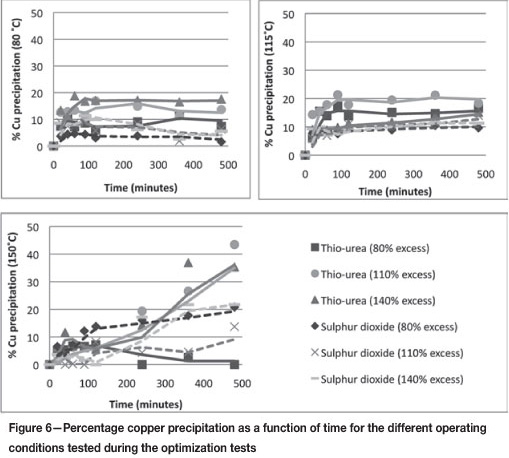
Copper and nickel precipitation should be limited as far as possible. Figure 6 shows the percentage copper precipitation as a function of time for the different operating conditions investigated during the optimization tests. Similar trends were observed for the nickel, and these graphs are thus omitted.
Overall, it seems that the amount of copper and nickel precipitated by thiourea is greater than the amount precipitated by SO2 under all operating conditions. It also appears as though the amount of copper and nickel precipitated with thiourea increases with both increasing temperature and increasing percentage excess reagent.
The maximum amount of copper precipitated with thiourea was, on average, 12.9 per cent at a temperature of 80°C for the different percentage excess values. An average nickel precipitation of 9.05 per cent was obtained at this temperature. For SO2, the average copper and nickel precipitation at this temperature was 4.5 and 3.0 per cent respectively. At 110°C, an average copper and nickel precipitation of 16.7 and 18.0 per cent respectively was achieved using thiourea, while SO2 resulted in percentage precipitation values of 11.6 and 9.9 per cent. Finally, at 150°C, thiourea precipitated 26.3 per cent copper and 24.8 per cent nickel. SO2, on the other hand, resulted in copper precipitation of 18.8 per cent and nickel precipitation of 14.7 per cent. From this it is evident that the amount of copper and nickel precipitated with thiourea at each temperature significantly exceeds the amount precipitated when SO2 was used.
A possible explanation for the greater precipitation of copper with thiourea may be related to the Te precipitation. If copper precipitates in a form that is not available for cementation with Te (as may be the case for thiourea), less of the precipitated copper is redissolved into solution. It is known, however, that SO2 allows the copper to precipitate in a form that is open to cementation. Therefore, more copper is redissolved when SO2 is used as a reagent, resulting in less copper precipitation.
Recalling the reaction mechanism for Rh precipitation (McGeorge et al., 2009), it can be noted that copper precipitates with the rhodium in the homogeneous reactions, heterogeneous growth, and during co-precipitation. It thus makes sense that a reduction in the amount of copper in the solution will occur. An examination of the copper precipitation trends shows that it may be possible to relate these to the rhodium precipitation trends as well. Since more Cu is precipitated by thiourea, more Cu is available for cationic substitution by the rhodium in solution. Lower copper precipitation by SO2 means that the less Cu is available in a form where Rh substitution can take place. This factor could be contributing to the fact that lower rhodium precipitation is achieved with SO2 than with thiourea.
Practical considerations
It is important to take the practical implications associated with the use of each reagent on the plant into account before choosing a reagent for implementation.
Thiourea is often used in electrowinning as a grain refiner (Hiskey et al., 1998) and is thus present in the process already. However, it is not known what effects this reagent will have on the process when it is added upstream of the electrowinning section. This is due to the fact that it is unclear which solid and dissolved species form during rhodium precipitation with this reagent. Solid-liquid separation may be difficult to achieve, and both solid and dissolved species may have detrimental effects on the process operation.
SO2 is the reagent currently used in the Se/Te removal section of the plant, and would thus probably require fewer modifications to implement than thiourea. It may thus be cheaper to use than thiourea. This reagent is, however difficult to manufacture and has major health and safety risks associated with it, unlike thiourea. For this reason, implementing thiourea may be beneficial, despite the higher capital costs.
Introducing larger quantities of sulphur generally decreases the size of the metal sulphide particles formed. This may lead to difficulties in solid-liquid separation if too much of either reagent is added.
Conclusions
From a practical and technical feasibility point of view, thio-urea and SO2 were deemed to be the most suitable reagents to be utilized for Rh precipitation from the pregnant copper sulphate leach solution. In general, when using thiourea as precipitation reagent, Rh precipitation and Se precipitation in excess of 90 per cent were obtained. However, little tellurium precipitation was achieved when using this reagent. In addition, the undesirable precipitation of copper and nickel occurs to a larger extent than when using SO2.
It should also be noted that the solid and dissolved species formed using thiourea are unknown and thus the effects of these compounds on the process are as yet undetermined. For this reason, it is recommended that a solids analysis is performed on the precipitate formed using this reagent. Investigations would also have to be performed on the dissolved species formed. To increase the amount of Te precipitated with thiourea, one could also vary the operating conditions for future tests. In this case, it would be necessary to carefully monitor the effects of these operating conditions on the precipitation of copper and nickel.
Although less Rh precipitation was obtained when using SO2 compared to thiourea, SO2 achieved Se and Te precipitation in excess of 80 per cent. Additionally, this reagent allows for limited precipitation of copper and nickel. It has, however, been observed that the Rh precipitation properties of this reagent improve at higher temperatures and greater percentage excess values. It is thus recommended that further studies be done on the operating conditions that will allow the highest Rh precipitation to take place when using SO2.
To determine which of these reagents would be best in a practical environment, it would also be necessary to perform a cost analysis for each reagent. This analysis would have to weigh the decreased manufacturing and safety precaution costs associated with thiourea against the capital cost of modifying the process to accommodate this reagent. This would then have to be compared to the manufacturing and modified operating costs associated with using a sulphur dioxide reagent.
Acknowledgements
The author would like to thank Western Platinum Limited (a subsidiary company of Lonmin Plc) for permission to publish the paper, as well as for financial and technical assistance provided.
References
1. DORFLING, C., AKDOGAN, G., BRADSHAW, S.M., and EKSTEEN, J.J. 2011. Determination of the relative leaching kinetics of Cu, Rh, Ru, and Ir during the sulphuric acid pressure leaching of leach residue derived from Ni-Cu converter matte enriched in platinum group metals. Minerals Engineering, vol. 24. pp. 583-589. [ Links ]
2. HABASHI, F. 1999. Textbook of hydrometallurgy, second edition, Métallurgie extractive Quebec. [ Links ]
3. HARAÑCZYK, I., SZAFIRSKY, B., and FITZNER, K. 2002. The influence of the rate of selenium crystallization from aqueous solutions on its morphology. Journal of Mining an Metallurgy, vol. 38. pp. 33-48. [ Links ]
4. HISKEY, J.B. and CHENG, X. 1998. Fundamental studies of copper anode passivation during electrorefining: Part III. The effect of thio-urea. Metallurgical and Materials Transactions, vol. 29B. pp. 53-58. [ Links ]
5. HOFIREK, Z. 1983. Process for removing dissolved selenium values from an acidic aqueous copper sulphate solution, US pat. number: 4 377 556. [ Links ]
6. MCGEORGE, B., GAYLARD, P.G., and LEWIS, A.E. 2009. Mechanism of rhodium(III) co-precipitation with copper sulphide (at low Rh concentrations) incorporating a new cationic substitution reaction path. Hydrometallurgy, vol. 96. pp. 235-245. [ Links ]
7. MENSAH-BINEY, R., Reid, K.J., and HEPWORTH, M.T. 1994. The loading capacity of selected cation exchange resins and activated carbons for gold-thiourea complex. Minerals Engineering, vol. 8. pp. 125-146. [ Links ]
8. SHERRITT GORDON MINES LIMITED, 1983. Base Metal Refinery: Selenium Removal and Reside Treatment Operating Manual. [ Links ]
9. SHIBASAKI, T., ABE, K., And TAKEUCHI, H. 1992. Recovery of tellurium from decopperizing leach solution of copper refinery slimes by a fixed bed reactor. Hydrometallurgy, vol. 29. pp. 399-412. [ Links ]
10. SÖHNEL, O. and GARSIDE, J. 1992, Precipitation: Basic Principles and Industrial Applications, Butterworth-Heinemann Ltd. [ Links ]
11. WEIR, D.R., VOSAHLO, E.A., and GENIK-SAS-BEREZOWSKY, R.M. 1980. Removal of selenium from sulphate solutions, US pat. number: 4 222 999. [ Links ]
12. WEIR, D.R., KERFOOT, D.G.E., and SCHEIE, H.C. 1982. Removal of Selenium (IV) and (VI) from Acidic Copper Sulphate Solutions. US pat. number:´4 330 508. [ Links ]
13. WANG, S., WESSTROM, B., and FERNANDEZ, J. 2003. A novel process for the recovery of Te and Se from copper slimes autoclave leach solution. Journal of Minerals and Materials Characterization and Engineering, vol. 2. pp. 53-64. [ Links ]
Paper received Dec. 2011
Paper written on project work carried out in partial fulfilment of B. Eng (Chemical Engineering)














