Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 n.4 Johannesburg Apr. 2012
JOURNAL PAPERS
Leaching of rare earth elements from bentonite clay
J.G. van der Watt; F.B. Waanders
School of Chemical and Minerals Engineering, North-West University, Potchefstroom Campus, South Africa
SYNOPSIS
Due to increasing concerns of global rare earth element shortfalls in the near future, possible alternative sources of rare earth elements have recently become of economic interest. One such alternative is decanting acid mine water originating primarily from abandoned old mines in the Witwatersrand region of the Republic of South Africa. In this study, a novel way of rare earth element removal from the acid mine drainage was employed, making use of bentonite clay, which has very good adsorbent properties, as a rare earth element carrier material. The process can be economically viable only, if the elements can be selectively removed from the bentonite clay carrier material so as to yield reusable clay. Acid leaching was proposed to liberate the adsorbed rare earth elements from the bentonite clay. Accordingly, acid leaching experiments were performed to study the desorption of three commonly-found rare earth elements, namely neodymium, samarium, and dysprosium, from bentonite clay in the presence of sulphuric and hydrochloric acid. It was established that the three rare earth element species could be selectively removed as a group from iron, magnesium, and manganese metals through the careful manipulation of the pH. An investigation into the kinetic aspects of the rare earth element desorption process from the bentonite clay was also undertaken. The applicability of various kinetic models such as zero-order, pseudo first-order, pseudo second-order, elovich, parabolic diffusion, and power function were tested to describe the time-dependent desorption of rare earth elements from bentonite clay. It was determined that the pseudo second-order kinetic model represented the dissolution processes for neodymium, samarium, and dysprosium from bentonite clay the most accurately.
Keywords: rare earth elements, REE, bentonite clay, acid mine drainage, AMD, leaching, removal.
Introduction
Acid mine drainage (AMD) is an extremely serious form of pollution that is of growing concern throughout various parts of the world. The presence of AMD can lead to the leaching of toxic heavy metals from underground and surface workings. Some of the most dangerous toxic heavy metals that can be liberated by the highly acidic waters include arsenic, lead, mercury, cadmium, chromium, and aluminium. The increased toxic heavy metal concen- trations and low pH of the water can endanger the surrounding fauna and flora if it is discharged uncontrollably into rivers and other water systems. AMD that continuously overflows into nearby rivers is known to damage aquatic systems tens of kilometres downriver from the primary discharge site. The great deal of attention that has been paid to the dangers posed by the commonly known heavy metals has overshadowed the hazards posed by other species such as the rare earth elements (REE). The large scale use of REE in the electronic, optical, magnetic, catalytic, agricultural, medicine, and stockbreeding industries means that the REE can easily enter ecological systems and ultimately end up in the human body via the food chain and clinical treatment procedures 1-3.
The removal of REE from various aquatic systems has been studied previously, but to a lesser extent than the heavy metals. Chegrouche et al.4A studied the removal process of lanthanum from aqueous solutions by natural bentonite, and Nagasaki et al.5 examined the affinity of finely dispersed montmorillonite colloidal particles for americium and lanthanides. Both of these studies proved the feasibility of removing REE from aqueous systems with the use of bentonite clay. However, the problem associated with the reusability of the clay persists. By utilizing novel extraction methods, the heavy metals and REE can be liberated from the bentonite clay to yield reusable clay. The liberated metals can be refined and sold. This procedure allows for a sustainable, cost-effective, and environmentally friendly process.
Literature
REE in the Witwatersrand
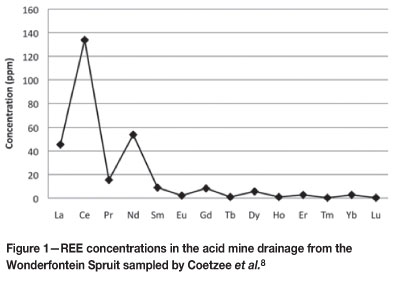
The Witwatersrand Basin in South Africa is a geological basin that spans roughly 350km and holds the largest gold reserves of any geological feature in the world. According to the findings of Rasmussen et al.6, metamorphic monazite and xenotime can be found in the Witwatersrand basin. Rasmussen et al.6 found monazite minerals in the Western Areas and Kloof mine regions of the Central Rand Group, and xenotime minerals in the Parktown Formation regions of the West Rand Group. The light rare earth elements (LREE) are predominantly associated with monazite and the heavy rare earth elements (HREE) are principally associated with xenotime7. Thus the presence of xenotime and monazite in the Witwatersrand basin means that REE can be found in the area, and the occurrence of AMD in the West and Central Rand areas unequivocally means that the REE are bound to be found in contaminated aqueous systems. Few studies have been conducted to analyse the entire range of REE occurrence in the aqueous systems of the Witwatersrand Basin. Coetzee et al.8 examined the full spectrum of REE occurrence in the Wonderfontein Spruit near Carletonville on the West Rand. Figure 1 indicates the average concentration of the REE in the Wonderfontein Spruit. The data is, however, from the year 2001, and since then there have been countless efforts made to control the pH of the AMD with quicklime (CaO). Thus the precipitation of the REE caused by the quicklime will result in lowered concentrations of REE in the waters of the Wonderfontein Spruit. Figure 1 illustrates the higher concentration of the LREE in comparison to the HREE, which is indicative of the presence of monazite in the Central and West Rand areas.
Desorption kinetics of REE from bentonite clay
In contrast to the vast amounts of literature related to the kinetics of metal ion adsorption onto bentonite clay, the literature on the desorption kinetics of metals ions from bentonite clay is limited. In order to examine the kinetics of rare earth element desorption from bentonite clay, it is necessary to determine the suitability of different desorption kinetic models. The most frequently used models for desorption include the zero-order, pseudo first-order, pseudo second-order, Elovich, power function, and parabolic diffusion equations9-12.
Havlin et al.13 studied the release kinetics of potassium from soils comprising montmorillonite-mica minerals. They found that it is possible to describe desorption kinetics by means of uncomplicated single-term equations, instead of complex equations that contain three simultaneous first-order rate terms.
According to Shirvani et al.9, the pseudo second-order model satisfactorily represented the desorption of cadmium from silicate clay minerals with high surface areas and adsorption capacities. Because bentonite clay is a silicate clay, the desorption process can possibly be represented by pseudo second-order kinetics.
According to Tseng et al.14, it can be assumed that the desorption behaviour described by the pseudo second-order equation is controlled by a second-order reaction. The second-order reaction can be written as:

with qt and qe the amounts of ion species (ppm) desorbed at any point in time and at equilibrium conditions respectively. The symbol, k2 (ppm-1.time-1) is a rate constant. Integrating Equation [1] with the initial condition, qt = 0 at t= 0 (Equation [2]), results in Equation [3].
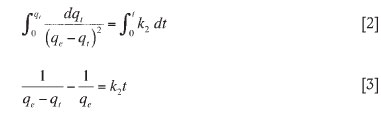
Rearranging Equation [3] yields:

It is difficult to construct a plot with Equation [4]. Equation [4] can rather be rewritten in a linear form as Equation [5]:

By plotting t/qt as a function of t, the values of qe and k2 can be computed. If the aforementioned values and constants are known, it is possible to predict the dissolution kinetics of the species at varying time periods.
Experimental Clay preparation
Due to the low concentrations of REE in the AMD, the concentrations of three representative REE species were increased to enable easier interpretation of the desorption results. Nd and Sm were chosen as the representative species for the LREE and Dy represented the HREE. The REE concentrations in 10 ℓAMD were elevated above 300 of ppm with the addition of Nd, Sm, and Dy nitrate salts. Bentonite clay was than added to the AMD the amount of bentonite being 7.5 weight per cent of the AMD mass. The pH of the slurry mixture was elevated to a value of 8 with the addition of CaO. The pH increase ensured complete removal of REE from the AMD. After 24 hours the water was carefully drained from the clay. The prepared clay was subsequently used in both the desorption and kinetic experiments.
Desorption experiments
Sulphuric and hydrochloric acid were used as lixiviants to leach the REE from the loaded bentonite clay. A pH range of 1.5 to 7 was selected to study the desorption of the REE from the clay in order to yield a good representation of the entire desorption spectrum. The clay samples were leached at 25°C for 60 minutes. After 60 minutes the slurry mixtures were filtered. The clay precipitate samples were sent for energy dispersive X-ray spectroscopy (EDS) analysis and the filtrate samples were analysed by inductively coupled plasma-mass spectrometry (ICP-MS).
Kinetic experiment
Only sulphuric acid was used as a lixiviant to study the time-dependent desorption of REE from the prepared clay. The experiment was conducted at a pH value of 3. The slurry mixture was leached at 25°C for a period of 180 minutes. Samples were continually taken during the experiment and after 180 minutes the slurry mixture was filtered. The clay precipitate and filtrate samples were analysed by means of EDS and ICP-MS techniques respectively.
Results and discussion
REE fractionation during desorption
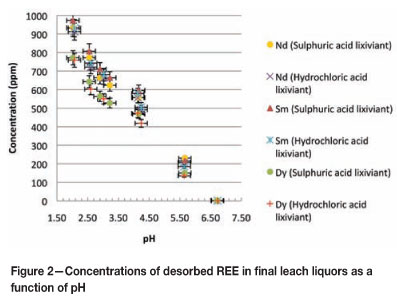
The leaching experiments that were carried out with both sulphuric and hydrochloric acids yielded comparable results with regard to leaching effectiveness and selectivity. Figure 2 (based on the ICP-MS results) shows the similarities between the experiments that utilized the different acid lixiviants. Error bars are included to indicate experimental errors in the ICP-MS results (5 per cent) and all the pH measurements include a standard deviation error of 0.2. It is clear from Figure 2 that increased desorption with increasing pH. Sample 1 in Table I indicates the amount of REE that was attached to the bentonite clay prior to leaching. The EDS results were expressed in terms of weight percentage of oxides. The EDS results of samples 2 and 3 indicate that as the atomic number of the REE increased, a slight increase in desorption was observed. No abrupt dissimilarity between the fractionation of the LREE from the HREE was observed. Similar findings have been described by Mihaljevic et al. 15,who reported that the HREE were preferentially released from bentonite clay at different solid/liquid ratios in a simulated wine purification process. The reason for the slight preferential removal of the HREE from the clay could be attributed to the phenomenon of lanthanide contraction. According to Biddau et al.16, the reduction in the ionic radii from La through to Lu, leads to a greater stability of the HREE in solution as opposed to the LREE. Although the phenomenon of lanthanide contraction was observed, it was not great enough to permit efficient separation of the LREE from the HREE. Instead, greater separation was observed between the REE as a group and other elemental species that commonly occur in AMD.
Selective removal of REE
The fractionation observed under hydrochloric and sulphuric acid leaching conditions for Nd, Sm, and Dy (Figure 2) was very similar. As a result, only Sm will be used as representative REE to discuss the selective removal of REE from other metal species under sulphuric acid leaching conditions.
Fractionation between samarium and iron
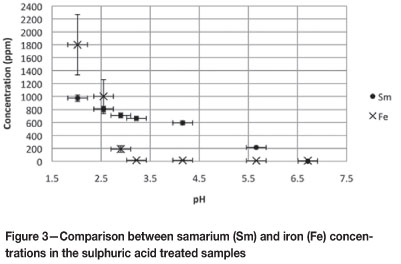
Figure 3 indicates the concentrations of Sm and iron (Fe) that were liberated from the clay under sulphuric acid leaching conditions. It is apparent that the REE could be selectively leached from the clay with minimal liberation of Fe between pH values of 3.0 and 4.2. Similar Fe desorption results were obtained by Enslin et al.17. Enslin et al.17 indicated that it was possible to selectively remove Fe from the actinide uranium (U) with sulphuric acid at a pH value of 3.0. The superior desorption of Fe above that of Sm and U at pH values of 3.0 suggests that the bonding characteristics of the lanthanides and actinides onto bentonite clay are very similar.
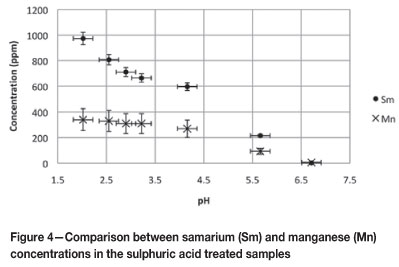
Fractionation between samarium and manganese
Figure 4 indicates the concentrations of Sm and manganese (Mn) that were liberated from the clay under sulphuric acid leaching conditions. The Sm could be separated from Mn at a pH value of 2.0, albeit not very effectively. The findings by Enslin et al. 17 also showed that it was difficult to selectively remove U from Mn at a pH value of 2.0, confirming the idea that the actinides and lanthanides share similar clay bonding characteristics.
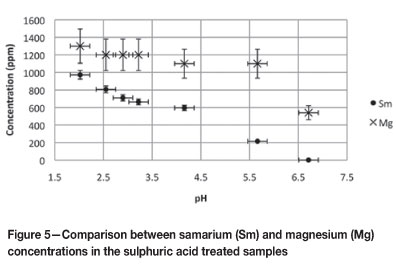
Fractionation between samarium and magnesium
Figure 5 indicates the concentrations of Sm and magnesium (Mg) that were liberated from the clay under sulphuric acid leaching conditions. It is evident that Mg was easily removed from the bentonite clay with minimal liberation of Sm. Optimal separation between Sm and Mg was obtained between the pH values of 5.5 and 6.7.
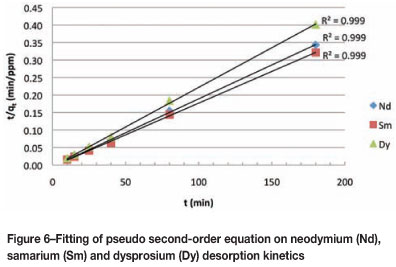
REE desorption kinetics
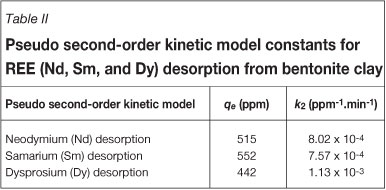
Six kinetic models were investigated to explain the desorption kinetics of the REE from bentonite clay. It was apparent that the pseudo second-order kinetic model yielded the best overall representation for the desorption reactions of the REE (Nd, Sm, and Dy) from bentonite clay as a result of the high coefficient of determination (R2) values. The R2 values served as an indication of the proportionate variation in the observed concentration values that can be explained by the linear relationship between time and concentration. Figure 6 indicates the linear trend for the pseudo second-order kinetic model based on Nd, Sm, and Dy desorption from bentonite clay with time. The R2 values of the pseudo second-order model were greater than 0.99, whereas the R2 values of all the other kinetic models were less than 0.80. The pseudo second-order kinetic model is reminiscent of desorption from siliceous clays. This kinetic experiment concurs well with the findings reported by Sen and Gomez18 and Tseng et al.14, of whom both reported that adsorption and desorption processes on siliceous clays follow pseudo second-order kinetics. The final equilibrium concentrations (qe) and rate constants (k2) for the pseudo second-order model are summarized in Table II. The equilibrium concentrations and rate constants, based on the three different REE species, are all very similar and confirm the negligible fractionation between the LREE and the HREE.
Conclusions
It was established that the three rare earth element species Nd, Sm, and Dy could be selectively removed as a group from Fe, Mg, and Mn metal species. Between pH values of 3.0 and 4.2, the three rare earth element species could be selectively removed from the clay without dissolution of Fe. At pH values in excess of 5.5, Mg could be selectively removed from the clay with minimal desorption of rare earth elements. The removal of Mn from the rare earth elements was possible, but not as effectively as compared to the Mg and Fe species. The optimum pH at which the Mn could be removed from the rare earth elements was 2.0. It was, however, not possible to selectively remove the rare earth elements from one another in two distinct groups classified as the LREE and the HREE. Although fractionation between the LREE and HREE occurred under acid leaching conditions, the differences in rare earth element desorption was marginally small when examined comparatively. The small amount of fractionation that occurred during acid leaching can be attributed to lanthanide contraction. It was observed that the efficiency of rare earth element desorption increased with a decrease in the rare earth element atomic radii.
The study of the time-dependent desorption of rare earth elements from bentonite clay revealed important information as to the characteristics of the bonds that occur between the rare earth elements and bentonite clay. It was observed that outer sphere adsorption can occur at pH values as low as 3.0, and that the inner sphere adsorption occurred predominantly in strong basic conditions. It can be concluded that the pseudo second-order kinetic model represented the dissolution processes of Nd, Sm, and Dy from bentonite clay most accurately, with R2 values in excess of 0.99. The chemical and physical similarities shared between the REE as a group signify that the pseudo second-order kinetic model will most definitely be able to describe the desorption of other REE from bentonite clay.
References
1. LUSTY, P. and WALTERS, A. Rare earth elements. British Geological Survey, Nottingham, UK, 2010. 45 pp. (Minerals Profile). [ Links ]
2. HE, X., ZHANG, Z., ZHANG, H., ZHAO, Y. and CHAI, Z. Neurotoxicological evaluation of long-term lanthanum chloride. Toxicological Sciences, vol. 103, 2008. pp. 354-361. [ Links ]
3. WEIDONG, Y., PING, Z., JIESHENG, L. and YANFANG, X. Effect of long-term intake of Y3+ in drinking water on gene expression in brains of rats. Journal of Rare Earths, vol. 24, 2006. pp. 369-373. [ Links ]
4. CHEGROUCHE, S., MELLAH, A., and TELMOUNE, S. Removal of lanthanum from aqueous solutions. Water Research, vol. 31, 1997. pp. 1733-1737. [ Links ]
5. NAGASAKI, S., TANAKAA, S. and SUZUKIA, A. Affinity of finely dispersed montmorillonite colloidal particles for. Journal of Nuclear Materials, vol. 244, 1997. pp. 29-35. [ Links ]
6. RASMUSSEN, B., FLETCHER, I.R., MUHLING, J.R., MUELLER, A.G. and HALL, G.C. Bushveld-aged fluid flow, peak metamorphism, and gold mobilization in the Witwatersrand basin, South Africa: Constraints from in situ SHRIMP u-Pb dating of monazite and xenotime. Geology, vol. 35, 2007. pp. 931-934. [ Links ]
7. CETINER, Z.S., WOOD, S.A. and GAMMONS, C.H. The aqueous geochemistry of the rare earth elements. Part XIV. The solubility of rare earth element phosphates from 23 to 150°C. Chemical Geology, vol. 217, 2005. pp. 147-169. [ Links ]
8. COETZEE, H., WINDE, F., and WADE, P.W. An assessment of sources, pathways, mechanisms and risks of current and potential future pollution of water and sediments in gold-mining areas of the Wonderfonteinspruit catchment. Report No. 1214/1/06. Water Research Commission, Pretoria, 2006. [ Links ]
9. SHIRVANI, M., SHARIATMADARI, H., and KALBASI, M. Kinetics of cadmium desorption from fibrous silicate clay minerals: Influence of organic ligands and aging. Applied Clay Science, vol. 37, 2007. pp. 175-184. [ Links ]
10. BASHIRI, H. Desorption kinetics at the solid/solution interface: a theoretical description by statistical rate theory for close-to-equilibrium systems. The Journal of Physical Chemistry, vol. 115, 2011. pp. 5732-5739. [ Links ]
11. WANG, D.Z., JIANG, X., RAO, W., and He, J.Z. Kinetics of soil cadmium desorption under simulated acid rain. Ecological Complexity, vol. 6, 2009. pp. 432-437. [ Links ]
12. Lü, X.N., XU, J.M., MA, W.Z., and Lu, Y.F. Comparison of seven kinetic equations for Ê release and application of kinetic parameters. Pedosphere, vol. 17, 2007. pp. 124-129. [ Links ]
13. HAVLIN, J.L., WESTFALL, D.G., and OLSEN, S.R. Mathematical Models for Potassium Release Kinetics in Calcareous Soils. Soil Science Society of America Journal, vol. 49, 1985. pp. 371-376. [ Links ]
14. TSENG, J.Y., CHANG, C.Y., CHANG, C.F., CHEN, Y.H., CHANG, C.C., JI, D.E., CHIU, C.Y., and CHIANG, P.C. Kinetics and equilibrium of desorption removal of copper from magnetic polymer adsorbent. Journal of Hazardous Materials, vol. 171, 2009. pp. 370-377. [ Links ]
15. MIHALJEVIC, M., ETTLER, V., HRADIL, D., ŠEBEK, 0., and STRAND, L. Dissolution of bentonite and release of rare earth elements at different solid/liquid ratios in a simulated wine purification process. Applied Clay Science, vol. 31, 2006. pp. 36-46. [ Links ]
16. BIDDAU, R., CIDU, R., and FRAU, F. Rare earth elements in waters from the albitite-bearing granodiorites of Central Sardinia, Italy. Chemical Geology, vol. 182, 2002. pp. 1-14. [ Links ]
17. ENSLIN, F., VAN DER MEY, L., and WAANDERS, F. Acid leaching of heavy metals from bentonite clay, used in the cleaning of acid mine drainage. Journal of The Southern African Institute of Mining and Metallurgy, vol. 110, 2010. pp. 187-191. [ Links ]
18. SEN,, T.K. and GOMEZ, D. Adsorption of zinc (Zn2+) from aqueous solution on natural bentonite. Desalination, vol. 267, 2011. pp. 286-294. [ Links ] ¨
Paper received Dec. 2011
Paper written on project work carried out in partial fulfilment of B. Eng (Chemical Engineering)














