Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 no.3 Johannesburg Mar. 2012
JOURNAL PAPERS
Pelletizing of Sishen concentrate
P. Mbele
Kumba Iron Ore, Johannesburg, South Africa
SYNOPSIS
Iron ore concentrate is not easily transported to, or processed in, ironmaking plants, therefore it is necessary to agglomerate. This paper will determine the possibility of agglomerating Sishen concentrate into suitable ironmaking feedstock.
Pelletizing is one of the conventional and preferred agglomeration processes for iron ore concentrate investigated at Kumba Value-In-Use Laboratories. Sishen concentrate was mixed with binder, and then pelletized using a laboratory pelletizing disc; afterwards the green pellets were indurated at high temperatures to give strength high enough to survive handling and lessen disintegration in the shaft during ironmaking.
Sishen pellets have satisfactory physical and metallurgical properties which make them suitable feedstock for the direct reduction processes. These were tested under Midrex shaft operational conditions and performed well, with a metallization of 94.47% being attained.
Keywords: pelletizing, pellets, Sishen concentrate, iron ore concentrate.
Introduction
Background
Sishen Mine is one of Kumba Iron Ore's mines, situated in Northern Cape Province.
During beneficiation (dense medium separation and jig plants) approximately 3.5 Mt/a of slimes material is produced and discarded to the tailings dam. This material has an iron (Fe) content of approximately 54%, which can be upgraded by means of two-stage SLon® magnetic separators1, to produce a high grade iron ore concentrate of 66.5% Fe.
This concentrate is not easily transported or processed in ironmaking plants; therefore it is necessary to agglomerate it. This test work was initiated to investigate the possibility of pelletizing Sishen concentrate into a suitable feedstock for ironmaking.
Agglomeration processes
There are a various agglomeration techniques for fine iron ore, namely: sintering, nodulizing, briquetting, and pelletizing.
Sintering is where the fuel and ore are combined and ignited to form porous agglomerates. Sinter is a popular feed for blast furnace operations; however, the drawback is that the concentrate in this form can be used only in very limited quantities at the sinter plants. Larger amounts of concentrate additions into the sinter mix adversely influence the bed permeability and hence the sinter production rate and product quality.
Nodulizing is the process where fine ore is tumbled in a rotary kiln at close to melting temperature. Drawbacks are that it is difficult to control the product size, the nodules have poor reducibility, and the process as a whole is costly in terms of energy consumption2.
Briquetting is one of the oldest iron ore agglomeration methods, but it has since been replaced by sintering and pelletizing. Briquetting is the process where a mixture of fine ore (concentrate) and binder is compressed in a briquetting die to form a product with uniform size and shape. Developments of briquetting techniques, such as hot briquetting, have yielded products of the same standards as those of pelletizing and sintering. The drawbacks of the process are that it is costly to maintain the worn briquetting dies, and has low production capacity.
Pelletizing is one of the preferred agglomeration processes for iron ore concentrate, as the chemical, physical, and metallurgical characteristics of pellets make them a more desirable feed for ironmaking processes. Pelletizing is an agglomeration technique where fine concentrate material is mixed with a binder and balled in a pelletizing disk, and then baked to acquire enough strength. Pellets are handled and transported without difficulty and are suitable feedstock for iron makers.
Pelletizing process
The pelletizing process was selected for this study due to its popularity in ironmaking business and the ability to produce uniform-sized pellets at high capacity and low cost.
Pellets are made from iron ore concentrates that have an average iron (Fe) content of at least 60% and particle size of 80%<75 µm. The concentrate material is mixed with a binder in an intensive mixer, then fed to the pelletizing disc for balling—while being moistened by a water spray system. Other materials, such as fluxes, can also be added into the concentrate and binder mixture if required. Moist fine material is then balled into green pellets and sieved separate a product -16 mm +10 mm in size.
The pellet nucleation initiates due to the surface tension forces of water and collision between particles. When the disc rotates, it causes the wetted fines to form a small seed-like 'nuclei' of particles which grow into pellets as they pick up loose grain particles during rotation of the disc. The angle of the disc determines the residence time, the pellet size, and the productivity of the pellet being formed.
The green pellets are dried, preheated, and baked in the furnace to improve physical strength and metallurgical properties, in order to survive handling and transportation without excessive dust generation and disintegration, and to be suitable for further ironmaking processes. The pellets are baked at a temperature of approximately 1300°C, lower than the melting temperature of iron ore, where the liquid phase formation of molten and semi-molten silicate spreads between iron ore grains and wets the surfaces of grains and sinters them.
The primary factor governing the strength of pellets is the type of binder used. The binder carries out two important functions in iron ore pelletizing. It makes the moist ore plastic so that it can nucleates seeds, that grow at a controlled rate into well-formed pellets, and during drying it holds the particle in the agglomerate together while the water is removed, and continues to bind the particles until the pellet is heated sufficiently to sinter the grains together. The suitability of a binder is determined by how well it can carry out these functions, while at the same time not causing contamination of the pellet2.
Pelletizing test work
Raw materials characterization
Raw materials characterization is critical for the pelletizing process. The concentrate, together with other additives such as bentonite and fine coke, should meet certain specifications before it can be regarded as suitable pellet feed. Representative samples of all raw materials were characterized in the 'as-received' state to determine the chemical composition and particle size distribution.
Table I illustrates the chemical composition of all raw materials used during pelletizing test work. Sishen concentrate has a grade of 66.9% Fe and silica content of 2.2%, which makes it suitable for pelletizing in terms of chemical composition.
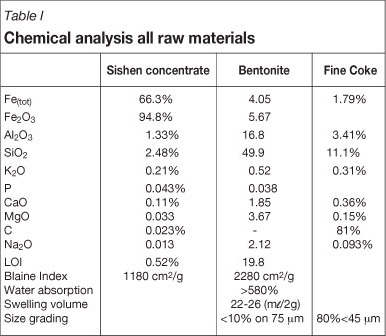
Bentonite binder
Although many binders have been proposed, such as ferrous sulphate, asphalt, starches, calcium and sodium compounds, and certain polymers, in practice bentonite is the most commonly used. Bentonite is a naturally occurring clay found in deposits of varying qualities around the world. It is formed by the hydrothermal alteration of volcanic ash deposits, consisting predominantly of minerals of the smectite group. The dominant smectite mineral is montmorillonite.
Bentonite is composed of discrete platelets that are separated by an exchangeable cation layer that has the capacity to absorb water and expand, thus controlling the moisture content of green and finished pellets. The water absorption behaviour of bentonite varies depending on whether the exchangeable cations are mostly sodium or calcium3.
Bentonite significantly improves physical properties, i.e. green and baked compressive strength, by two mechanisms. Firstly, it provides a source of colloidal material that decreases interparticle distances and thus increases Van der Waals forces. Secondly, it forms a solid bridge of hardened gel that strengthens particle contact points. The increased viscosity of fluids between the mineral grains in the pellets results in the production of the well-rounded, plastic pellets that can be easily handled for sizing and transport in the plant.
However, there are some drawbacks to the use of bentonite; the most remarkable is the contamination of the product with gangue silica4. Some forms of sodium bentonite are known to contain more than 65% SiO2 by weight. This additional silica blocks the porosity of the pellet, inhibiting the flow of reducing gases into the core of the pellet. This lowers the pellet reducibility, which in turn increases the energy requirements in the steel making process and the costs for handling and disposal of increased slag levelss.
Sodium-activated calcium bentonite was used as the sole binding agent during pelletizing testwork.
Sodium activation affects bentonite properties in two ways: firstly, by changing the water adsorption capacity and thus the swelling of bentonite gel, which improves the pellet quality; and secondly, by improving the dispersion of bentonite in the pellet matrix6.
Fine coke was used with the aim of improving the porosity of pellets, and subsequently their reducibility. Fine coke combusts during baking under an oxidizing atmosphere and forms pellets with a porous structure. Fine coke has high dry fixed carbon (DFC) of 81%, and low ash content, and is therefore suitable for the pelletizing process.
Particle size and specific surface area (Blaine Index value) of Sishen concentrate play a key role in the pelletizing process. These two material properties are most important during the formation of seeds and growing of pellets by adherence of loose fine material.
Normally, any material considered for pelletizing should have a specific area (Blaine Index value) >1200 cm2/g for proper balling characteristics7. The Blaine Index test of Sishen concentrate material resulted in an average value of 1180 cm2/g, slightly below the generally accepted value of 1200 cm2/g for pelletizing feed.
Material fineness is optimal for pelletizing however, the aim of this testwork was to determine the possibility of pelletizing Sishen concentrate material 'as-received', without additional milling, which is costly.
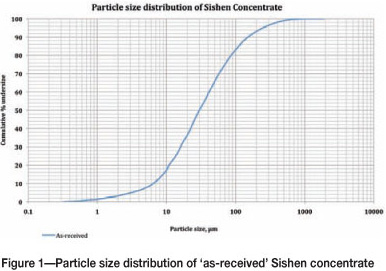
The particle size analysis for 'as-received' material was conducted on a laser diffraction size analyser, and the results are shown in Figure 1. The graph shows that the 'as-received' sample is 80%< 75 µm, which is a suitable particle size for pelletizing.
Laboratory scale pelletizing process
Pelletizing tests were conducted at the Value In Use Laboratories at Kumba Iron Ore. The following sub-processes were conducted to complete the laboratory test work.
Mixing
The purpose of the mixing process is to intensively mix Sishen concentrate with binder and/or other additives such as fine coke in order to form an admixture with a homogenous distribution of the various components yielding good green pellet formation and strength. The mixing recipes for the test work are shown in Table II.
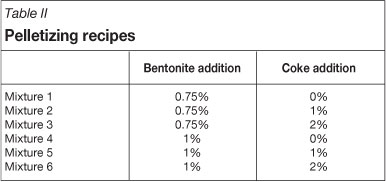
Sishen concentrate, binder, and fine coke were added to the high intensity mixer for 30 seconds. Binder and coke dosages were reported as mass percentages of Sishen concentrate. The general binder additions in iron ore pelletizing range from 0.5% to 1%; however, in this study only 0.75% and 1% were selected because these are typical binder dosages for normal operations. Coke additions were varied from 0% to 2% to determine the optimum coke addition that can yield adequate porosity.
Pelletizing process
A small amount of the pellet mixture material was then added to the pelletizing disk rotating at 60 r/min and at an angle of 70°, to create pellet 'seeds'. The seeds were moistened with water spray to retain moisture content while adding additional material to grow them into larger pellets. The pellets were removed from the drum periodically, and screened to control the desired size between -16 +10 mm. The water addition is well controlled to achieve the moisture content above 8%. The wet green pellets were then dried under atmospheric environment for 48 hours to remove all moisture.
Baking/induration process
Pellets require good physical and metallurgical properties to be suitable for transportation and further downstream ironmaking processes. To achieve these properties, the dry green pellets were indurated at 1300°C under an oxidizing atmosphere, following a specified heating pattern.
Evaluation tests and analysis of results
Physical properties of pellets
Moisture analysis
Immediately after production, 500 g of the pellets were dried at 105°C for 2 hours. After drying they were weighed in order to determine the weight loss, and the percentage of moisture was calculated.
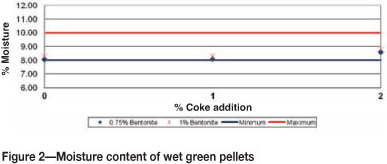
Figure 2 shows the moisture content of wet green pellets that were sampled immediately after pelletizing. The results showed that the bentonite binder effectively absorbed and retained moisture during pelletizing within acceptable levels of 8-10%. Suitable moisture levels are required to avoid unnecessary breakage of weak pellets (if they are too dry), or formation of a muddy mixture (if too wet).
Drop number
This test was conducted to determine if pellets will withstand dropping during pellet handling. Drop number testing was conducted by taking twenty pellets from the pelletizing disc and dropping them from a height of approximately one metre, and counting the number of drops from that height before a pellet broke or became deformed.
The drop number for Sishen pellets was low, approximately 2, because of low moisture levels and bentonite dosages. Satisfactory pellets normally have a wet drop number of >5. This means that Sishen wet green pellets are not elastic; they can easily break down during handling. The literature shows that the drop number increases linearly with moisture contents and also with higher binder dosages. The drop number of wet green pellets can be improved by increasing the moisture of the pellets to >9%, as binder dosages are relatively high already.
Wet green strength
This test determined the strength of the wet green pellets during handling (at the pelletizing section). When pellets collide with each other they should not deform or break.
Wet strength was evaluated by using the Instron compressive tester, and the load required to fracture the pellet was captured. The test was repeated for twenty pellets and the average strength was recorded. The wet green strength of the pellets was low, less than 10 N, for all pellet mixtures.
Dry strength of green pellets
This test was used to determine the strength of dry pellets to ensure that they will not degrade during handling after drying, and to measure the integrity of the binder after moisture has been removed. Dry strength of dry green pellets was evaluated by using an Instron compressive tester after the pellets had been dried in atmospheric air for two days. Twenty pellets were tested and the average strength recorded.
Industrially acceptable pellets have a dry green strength greater than 22 N before firing. Figure 3 shows the strength of dry green pellets was below the acceptable levels due to low binder additions. Dry green pellets are not required to withstand much handling.
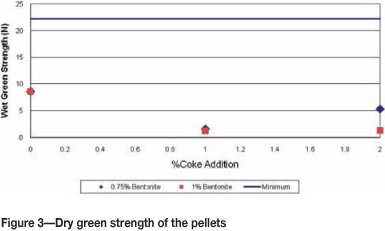
The low drop number and wet and dry green strengths could cause the degradation of pellets during processing in the pellet plant due to low Bentonite additions. Excessive handling of pellets before the baking process should be limited to minimize this risk. Bentonite binder is the best binder, yielding optimal strength even at low binder levels, although, only after firing.
In normal operations the pellets are handled wet and fed to the heating furnace, then dried and baked without too much handling. The pellets are discharged from the furnace strong enough to be transported and handled without any degradation.
Compressive strength of baked pellets
This test was conducted to determine the strength of baked pellets. The acceptable strength is 2000 N for direct reduction. The compressive strength of pellets was evaluated using the
Instron compressive strength tester after the pellets had been baked in the furnace at 1290°C. Twenty pellets were tested and the average strength was recorded.
The pellets were tested for compressive strength after baking for 10 and 15 minutes, and the results are shown in Figure 4 and 5.
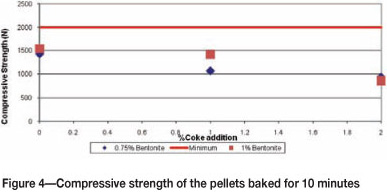
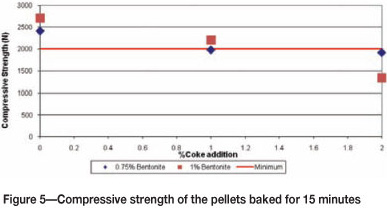
Figure 4 shows that pellets that were baked for 10 minutes did not achieve the required strength of 2000 N. This implies that the retention time of the pellets was not sufficient for slag bonding, recrystallization, and grain growth.
Figure 5 shows the compressive strength of pellets that were baked for 15 minutes, with 0.75% and 1% bentonite (without any coke addition), and 1% bentonite plus 1% coke addition. The pellets attained the required strengths within the specification of 2000N minimum.
Both Figures 4 and 5 reveal that as the amount of coke increases, the strength decreases. Addition of carbon in the form of coke lowers the melting point of the slag phase, resulting in excessive formation of the brittle binding phase, which causes weakening of the pellet structure.
The retention time of pellets on-temperature, 1290°C, which is just below melting temperature, is critical for slag bonding, recrystallization, and grain growth. Recrystallization is an important factor for the development of pellet strength. During slag bonding, the liquid phase formation of molten and semi-molten silicate has to spread between iron ore grains and wet the surfaces of the grains and sinter them.
Only the pellets that attained the required compressive strength were selected for further metallurgical testing
Porosity testing of baked pellets
Porosity is also an important physical property of pellets because it affects their reducibility. An increase in porosity will lead to a better reducibility of the pellets, but will cause a decrease in the pellet strength. The porosity tests were conducted on pellets made with 0.75% bentonite to determine the effect of coke addition on pellet porosity.
GeoPyc 1360 and Pycnometry instruments were used for the porosity measurements. In the GeoPyc instrument, the sample volume was analysed by packing it in silica sand, DryFlo. First, the sample chamber was filled with sand only and packed under rotating movement to desired pressure. From the position of the piston, the volume of the sand was calculated (blank measurement). Thereafter, the sample chamber was opened and the weighed sample was transferred inside. The packing to desired pressure was repeated and the total volume of sand and sample was measured (sample measurement). From the difference in volume between the sample and blank measurements, the sample envelope volume was calculated. The porosity was calculated by subtracting the volume of particles from the envelope volume according to Equation [1]. A sample chamber of 38.1 mm size was used for measurement. The standard packing pressure of 90 N for this chamber was used9.

where
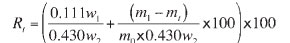
ε = fractional porosity
Ve = envelope volume
m = sample mass
ρα = absolute density (particle density).
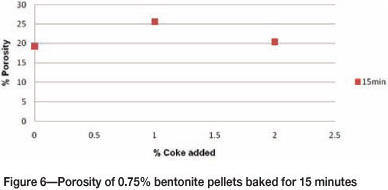
Figure 6 shows the porosity of 0.75% bentonite pellets with varying coke additions. When 1% coke was added, the porosity of pellets increased due to the combustion of carbon during baking. The porosity dropped with an increase in coke addition to 2%. One possible reason is that carbon increases the formation of low-melting iron silicate phases by lowering their melting point, thus blinding the pores of the grain structure (refer also to the chemical analysis in Table III). Pellets with low porosity cannot be reduced easily during the ironmaking process, ultimately affecting productivity. Coke can be added to the pellet to improve the porosity, but the addition is limited to 1% in this case.
Chemical analysis of pellets
Table III shows the chemical analysis of all pellet mixtures that were produced, and which have an acceptable Fe content of 66% minimum, which is the set specification for direct reduction processes. Pellets made with 0.75% bentonite (without any coke addition) had an acceptable Fe content and had the lowest silica content, which maked them more suitable for further iron making processes.
Mineralogical analysis of pellets
Baked pellets were examined by optical microscopy to determine the effect of longer induration time and coke addition on the mineralogical structure.
The micrographs in Figures 7-10 show the mineralogical analysis of the baked pellets.

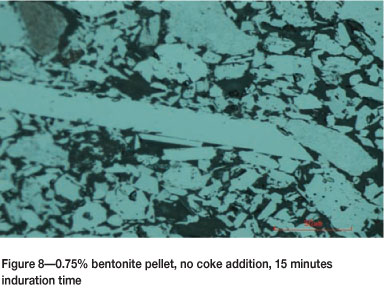


Figure 7 shows the structure of 0.75% bentonite pellets (without any coke addition) that were baked for 10 minutes, and which did not attain the required strength. The structure consists mainly of haematite (white phase) and the binding phase (dark phase). The grains had not yet recrystallized and there was no noticeable grain growth.
For the pellets baked for 15 minutes, the grains had fully recrystallized and acquired enough grain growth, as shown in Figure 8. Recrystallization is an important factor for the development of pellet strength, hence the pellets baked for 15 minutes were stronger than those baked for 10 minutes.
Addition of coke to the pellet mixture influences the microstructure of the baked pellets. During the baking process the haematite is reduced to magnetite to a certain extent, depending on the amount of coke added. Figures 9 and 10 show that the pellets contain a magnetite phase (grey); it is even more noticeable with 2% coke addition.
Addition of carbon in the form of coke lowers the melting point of the slag phase, resulting in increased formation of the binding phase which causes weakening of the pellet structure. Figure 10 shows that the grains have completely recrystallized and there is pronounced grain growth. Excessive binding phase and grain growth contribute to the low pellet strength and porosity. Coke addition can be an option, based on the fact that there was least improvement in pellet porosity when coke was added; pellets without coke had good porosity.
Metallurgical properties of pellets
Metallurgical properties were determined in order to give an indication of the behaviour of the pellets during reduction. The properties of the materials are important for energy consumption, smooth operation and the quality of the product. The properties tested were reducibility, reduction, disintegration, tendency to swell, and sticking tendency10.
Reducibility index (RI) of pellets - ISO 469511
This test method measured the ease with which the oxygen combined with iron in the baked pellets could be removed. It also made use of isothermal reduction of the test sample on a fixed bed at 950°C, using reducing gases consisting of 40% CO and 60% N2. During the test the sample was weighed for mass loss at specified intervals. The RI was calculated at an atomic ratio O/Fe of 0.9. Calculation for the degree of reduction after time was as follows:
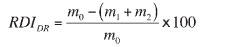
where
mo = mass of sample, in grams
m1 = mass of sample before reduction, in grams
mt = mass of test sample after reduction time t
w1 = iron (II) oxide as % by mass of test sample before test
w2 = total iron content as % by mass of the test sample before test.
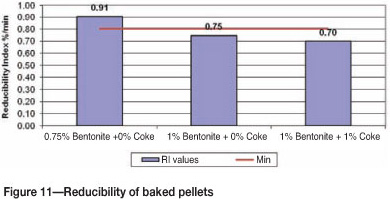
The general acceptable range for RI values is 0.8%/min to 1.4%/min. Figure 11 indicates that pellets made out of 0.75% bentonite had the highest reducibility of 0.91%/min on average, which is acceptable for ironmaking processes.
Further metallurgical tests were conducted on 0.75% bentonite pellets, since these displayed very good compressive strength and RI values. Operationally, that mixture can produce cost-effective pellets in terms of binder consumption.
Reduction disintegration index (RDI) - ISO 1125712
During reduction, pellets and other feedstock to the furnace are prone to low temperature breakdown (LTB) at temperatures between 400°C and 700°C. This test characterized the low temperature reduction disintegration index and degree of metallization for gas reforming processes (direct reduction application). The test employed isothermal reduction of the test portion in a Linder furnace (externally heated horizontal rotary tube) at 760°C using reducing gases consisting of CO, CO2, H2, and CH4. The sample was exposed to reducing conditions for 5 hours and was then cooled in an inert atmosphere.
Reduction -disintegration index (RDIdr)
The RDIDR expressed as a mass percentage of material less than 3.15 mm is calculated from the following equation:
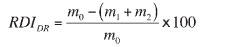
where
mo = mass of reduced test portion before sieving, including the dust trapped in the dust collector m1 = mass, in grams, of the fraction of the reduced test portion retained on the 10 mm sieve m2 = mass, in grams, of the fraction of the reduced test portion retained on the 3.15 mm sieve. As a general criterion for direct reduction pellets, the maximum fraction passing a 3.15 mm screen after reduction should be 5%. During testing at VIU laboratories, a RDIDR value of 4.10% was achieved for 0.75% bentonite pellets (without any coke addition), which is acceptable for direct reduction processes.
Degree of metallization (M)
The degree of metallization, M, expressed as a percentage by mass, is calculated from the following equation:
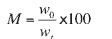
where
wo = metallic iron content, expressed as a percentage by mass, of the reduced test portion
wt= total iron content, expressed as a percentage by mass, of the reduced test portion.
The general criterion for direct reduction acceptance is 92% metallization. At VIU laboratories the degree of metallization for 0.75% bentonite pellet (without any coke addition) was 93% during testing, which is acceptable for direct reduction processes.
Swelling index - ISO 469813
At higher temperatures, it is very likely that the pellet's volume may increase beyond its initial volume. The volume increase is known as swelling, and it decreases the voids in the charge and thus impedes the gas flow. When swelling occurs pellets may crumble and gas permeability could be impaired.
In this test, the volume change that occurred during reduction of indurated pellets under unconstrained conditions was determined. Testing employed isothermal reduction of the sample at 900°C using 30% CO and 70%N2. The volume of pellets before and after reduction was measured. The free swelling index is expressed as a percentage using the formula:
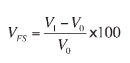
where
V0 = volume in cubic millimetres of the test sample before reduction
V1 = volume, in cubic millimetres of the test sample after reduction.
The general criterion is that swelling higher than 25-30% is undesirable. A swelling index of 7.89%, which is very low, was measured for 0.75% bentonite pellets. This illustrated that the baked pellets comprising 0.75% bentonite will not swell excessively beyond their initial volume during the ironmaking process.
Clustering index of pellets - ISO 1125614
Pellets have the tendency to form clusters at higher temperatures. This sticking tendency affects the maximum bustle temperature that could be used for DRI production, which determines the overall productivity that could be attained in the shaft. Sticking increases as the Fe content increases and gangue decreases, therefore high quality DR pellets have a high sticking tendency.
This test was conducted to determine the clustering of iron ore pellets as shaft direct-reduction feedstock. The test sample was isothermally reduced in a fixed bed at 850°C under static load, using a reducing gas consisting of H2, CO, CO2, and N2, until a degree of reduction of 95% was attained. The reduced test sample (cluster) was disaggregated by tumbling, using a specific tumble drum. The clustering index was calculated as the mass of clustered material accumulated after specified disaggregation operations.
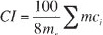
where
mr = total mass, in grams, of the test portion after the reduction
mc,i = mass, in grams, of the clustered material after the ith disaggregation operations.
Two clustering index tests revealed that these pellets do not form clusters under high temperature. This further verifies the good quality of these pellets.
Industrial basket test results
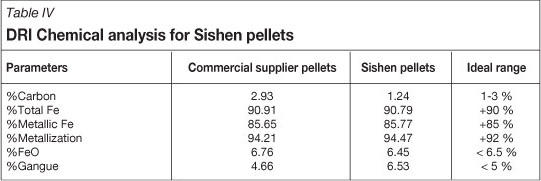
A batch of 0.75% bentonite Sishen pellets was sent to a Midrex Shaft for basket testing, to determine whether the produced pellets are suitable for the direct reduction process, and to determine their reduction properties. Sishen pellets were compared to those (from another supplier) currently being utilized in the Midrex Shaft. Both pellet baskets were exposed to normal operating conditions in the Midrex Shaft. The baskets were then removed and DRI material was analysed chemically.
Table IV shows the chemical analysis results for DRI produced from both pellet types. Both results were within the acceptable ideal range for direct reduction process, although the degree of metallization of the Sishen pellets exceeded that of the currently used pellets. This means simply that Sishen pellets are suitable for the ironmaking process because of high metallization their >93%, which is the main productivity driver for the direct reduction process.
Conclusion
The 'as-received' Sishen concentrate grade had an acceptable chemical composition with high Fe >66% content. The Blaine Index value was below the generally accepted value; however, if a high Blaine Index value is required additional milling should be considered
The chemical composition of pellets made with 0.75% bentonite (without coke addition) was acceptable at a high iron content, >66%, and the physical properties were suitable for the direct reduction process, since the pellets attained a satisfactory compressive strength, >2000 N. The metallurgical tests were also fully acceptable, with a good RI value of 0.91%/min, minimum swelling index, and no clustering of pellets occurred during testwork.
During industrial basket tests, Sishen pellets containing 0.75% bentonite displayed reduction properties that were within the acceptable ideal range for direct reduction processes.
All the pelletizing and evaluation test work showed that Sishen concentrate is a suitable pellet feed and can produce pellets with suitable pelletizing behaviour and satisfactory physical and metallurgical properties.
References
1. Skosana, D.J. Upgrading of Sishen thickener underflow slimes using the SLon® high gradient magnetic separator (HGMS). Iron Ore and Manganese Ore Metallurgy Conference, Johannesburg. The Southern African Institute of Mining and Metallurgy, 2011. pp. 59-70. [ Links ]
2. Kawatra, S.K. Binders in iron ore pelletization. Michigan Tech University, Michigan, 2008. pp. 7-9. [ Links ]
3. Kawatra, S.K. and Ripke, S.J. Laboratory studies for improving green ball strength in bentonite-bonded magnetite concentrate pellets. International Journal of Mineral Processing, vol. 72, nos 1-4, 2003. pp. 429-441 [ Links ]
4. Qiu, G., Jiang, T., Li, H., and Wang, D. Functions and molecular structure of organic binders for iron ore pelletisation. Colloids and Surfaces A: Physicochemical Engineering Aspects, vol. 224, no. 1, 29 August 2003. pp. 11-22. [ Links ]
5. akzonobel. Organic binder for iron ore concentrates. www.cs.akzonobel.com. 2009. [ Links ]
6. Saidi, A., Shamanian, M., Barati, M., and Azari, K. Hyperactivation of Bentonite in Pelletising Process. International Journal of ISSI, vol. 1, no. 1, 2 August 2004. pp. 38-41. [ Links ]
7. Poveromo, J. Industrial Minerals and Rocks, Metallurgical Uses -Agglomeration Processes- Pelletising and Sintering. pp. 6. [ Links ]
8. Forsmo, S.P.E., Apelqvist, A.J., Bjórkman, B.M.T., and Samskog, P.O. Binding mechanisms in wet iron ore green pellets with bentonite binder. vol. 169, no. 3, 13 November 2006, pp. 147-158 [ Links ]
9. Forsmo, S.P.E. and Vuori, J.P. The determination of porosity in iron ore green pellets by packing in silica sand. Powder Technology, vol. 159, no. 2, 2005. pp. 71-77. [ Links ]
10. Mashao, M.S. Evaluation of Iron Ore pellets and sinters for BF and DR use. Pelletising and Sintering in the ferroalloy and ironmaking industry Colloquium, Pretoria. The Southern African Institute of Mining and Metallurgy, 2008. pp. 4-7. [ Links ]
11. ISO 4695: Determination of reducibility by the rate of reduction index. 2007. [ Links ]
12. ISO 11257: Determination of disintegration and metallization of feedstock for direct reduction by gas reforming processes. 2007. [ Links ]
13. ISO 4698: Determination of relative free-swelling index. 1994. [ Links ]
14. ISO 11256: Determination of clustering index. 2007. [ Links ]














