Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 n.2 Johannesburg Jan. 2012
JOURNAL PAPER
Economics of mine water treatment
J. DvořáčekI; J. VidlářI; J. ŠtĕrbaI; S. HeviánkováI; M. VanĕkI; P. BartákII
IVŠB-Technical University of Ostrava, Czech Republic
IIKámen Ostromĕř, Czech Republic
SYNOPSIS
Mine water poses a significant problem in lignite coal mining. The drainage of mine water is the fundamental prerequisite of mining operations. Under the legislation of the Czech Republic, mine water that discharges into surface watercourse is subject to the permission of the state administration body in the water management sector. The permission also stipulates the limits for mine water pollution. Therefore, mine water has to be purified prior to discharge. Although all mining companies operate mine water treatment plants, there is hardly any utilization of the final effluent. The high expenditure involved in the more sophisticated methods of mine water treatment has fuelled the search for the commercial utilization of the effluent.
Keywords: mine water treatment, economics of treatment process, water utilization.
Introduction
Lignite coal ranks first among the mineral resources extracted in the Czech Republic. Opencast mines are the deepest sites in the landscape relief, and as such they are extremely sensitive to the inflow of both surface water and groundwater as well as rainfall water. Therefore, each opencast mine has to be equipped with a drainage system for controlled drainage of the mine water from the working area of the opencast mine. The definition of mine water is included in the Mining Act of the Czech Republic1. The Act also enables the mining company, among other things, to use the mine water free of charge and to discharge the excess amount.
The objective of the drainage is to ensure the smooth and safe running of an opencast mine. For this purpose, the Mining Act has been supplemented with other regulations of the State Mining Authority, the Water Act2, the Act on Environmental Impact Assessment3, etc. The above legal regulations deal not only with safety and technical issues of water drainage and operation of opencast mines, but also environmental protection, notably the protection of surface water and groundwater.
The drainage system of an opencast mine includes the safeguarding of the foreground against surface water and the drainage of the mine and the inside dump. The former involves the creation of drainage channels and, if required, watercourse rerouting in the prospective exploitation area. As a rule, the drainage of overburden benches and coal cuts is carried out by means of a system of open drainage channels. The exposed bed, i.e. the base of the prospective inside dump, is drained through a system of covered drainage channels. The water from the opencast mine and the inside dump drains into a reservoir that is sunk at the lowest part of the mine. The size of the reservoir has to be determined taking into consideration the local rainfall and water inflow. As a rule, the water is pumped from the reservoir by a pumping plant into the mine-water treatment plants. The final effluent is discharged into surface watercourses and the amount and quality are monitored as the method and conditions of mine water discharge into surface watercourses are set by the self-administration authorities. Water management practices that contravenes the Water Act are subject to penalization.
Technological process of mine water treatment
The area of our concern is two coal opencast lignite mines. As we will be discussing sensitive data on the costs of mine water treatment at the treatment plants of the respective mines, we consider it appropriate not to give a detailed description of the mines in question. They are situated in the northern part of the Czech Republic and will be referred to as Locality 1 and Locality 2.
Mine water accumulates in the lowest level of the mine and can be acid (pH 2.5-4) to neutral (pH 6-7). The acidity of water is the result of the biochemical dissolution process of iron disulphides contained in the coal. In order to decrease the corrosive action of mine waters, a pre-treatment is performed by partial neutralization, which is effected by dosing of lime wash to accumulation ponds.
The process continues with the sedimentation of suspended solids in large sedimentation tanks by means of gravity. Preliminary coagulation may also take place. The following stage of treatment involves oxidation to remove the soluble forms of iron and manganese from the water by means of aeration or addition of an oxidizing reagent- potassium permanganate. Controlled sedimentation is used to separate sludge from water. Thickening, dewatering via a centrifuge/filter press, and the disposal of sludge follow. The final effluent is then discharged into the surface watercourse.
However, this treatment process leaves high concentrations of sulphate ions (their concentration being between 600 to 1500 mg/l) unaffected, which means that the surface water lawful discharge limits are exceeded and that the water cannot even be utilized for industrial purposes.
The Faculty of Mining and Geology of the VŠB-Technical University of Ostrava, Czech Republic developed and tested on site a technology for the desulphation of mine water (Vidlář, et al.4-6) that enables the removal of sulphates as well as residual heavy metals. The chemical precipitation process is initiated by the addition of lime slurry and sodium aluminate. After flocculation, the resulting precipitate (the ettringite sludge) is dewatered in a filter press after its density has been increased by means of gravity settling. It is of advantage to utilize desulphation node sludge for the reactive precipitation of sulphates (aluminium ion donor) or to use it as silicate additive to improve the hydraulic properties of gypsum and lime mortars (Vidlář and Heviánková7). The output of the desulphation technology is demineralized (industrial, technological) water.
The precipitation reaction chemistry is based on analogy with the well-known reaction that takes place by hydration of ground clinker of calcium aluminate cements, which contain sulphates. An outcome of these heterogeneous chemical reactions is a new low-solubility mineral constituent, calcium aluminate sulphate (ettringite), which, as 'cement bacillus', was also identified by Bannister8 in 1936. Our desulphation method is based on the direct interaction of calcium and aluminium ions with sulphates present in water of high alkalinity (pH about 12.4).
From a theoretical point of view, the probable precipitation mechanisms are:
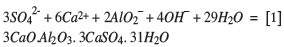
or also
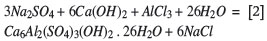
The calcium and hydroxyl ions for these reactions are provided by calcium hydrate, Ca(OH)2. It is of advantage to add the aluminium ions in the form of sodium aluminate, NaAlO2, which does not carry any other deleterious ions.
The removal of sulphates can be controlled to achieve desired contents, even below 300 mg/l.
The mine water desulphation technology of this project also provides for efficient removal of all Fe, Mn, and other metal ions, which occurs in the primary stage (high alkalization of input water by lime solution), when all metals present precipitate in the form of hydroxides. The second stage consists of sulphate precipitation, and the third stage provides for final neutralization of the purified water by liquid carbon dioxide, CO2 (Heviánková and Bestová9).
The desulphation process technology for mine water treatment is similar to the treatment process to produce potable water from water supply reservoirs, except for the final sanitation phase, i.e. disinfection.
Economics of the mine water treatment process
Current mine water treatment involves the following process: in Locality 1, mine water neutralization, removal of insoluble substances and partial removal of iron. In Locality 2, besides the removal of insoluble substances, the reduction of acidity of mine water and removal of manganese and iron are undertaken also. The purified mine water is discharged into the surface watercourse without further treatment nor utilization. One of the reasons is the high content of sulphate ions, which at present is not subject to any statutory requirements. This is expected to change in the coming years.
The extent of mine water treatment is the determinant of the unit costs. At the current Czech crown/US dollar exchange rate, the unit cost at Locality 1 is US$ 0.44/m3 and at Locality 2 US$ 1.20/m3 of treated mine water.
However, the final effluent has a high content of sulphates, and manganese concentration is not satisfactory either. It is only in Locality 2 that manganese is removed by oxidation using atmospheric oxygen. As the process becomes less efficient at lower mine water temperatures, potassium permanganate is used as oxidizing agent. However, it is added only when the limit concentrations of manganese in the effluent have been exceeded. Furthermore, if the optimum dose of oxidizing reagent is exceeded, the concentration of manganese in the effluent increases beyond the permissible level. The task force of the Faculty of Mining and Geology (Heviánková and Bestová9) proposed a modified process for removing high manganese levels from acid mine water. The process proposed includes alkalification using calcium hydroxide and chemical oxidation including neutralization by carbon dioxide.
However, the removal of sulphate ions and manganese from mine water involves higher operational costs.

The sulphates removal process entails the depreciation expense for buildings and technological equipment, higher operational costs, increased consumption of chemicals for water treatment, and capital lock-up in the inventory of chemicals. The estimated maximum capacity of the sulphates removal facility is 1 752 thousand cubic meters of treated mine water per year.
The manganese removal process involves greater consumption of chemicals, increased storage costs, increase in the depreciation expense for carbon dioxide storage, and higher freight cost.
At the current Czech crown/US dollar exchange rate, the estimated cost of sulphate removal from mine water is US$1.92 per cubic meter of treated mine water. The estimated cost of manganese removal using the technology proposed is US$0.10 per cubic meter of treated mine water.
Based on the above, it is possible to compare the unit costs of treated mine water using different treatment methods. The outcome is given in Table I. It is based on the actual data from the mine water treatment plants at both the localities, supplemented with the prospective methods of a more sophisticated mine water treatment process, i.e. removal of manganese and sulphates. Table I contains:
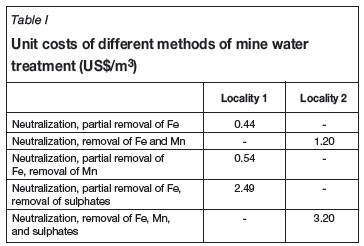
Actual cost of mine water treatment at Locality 1 and Locality 2
Actual cost of mine water treatment at Locality 1 plus the manganese removal proposal
Actual cost of mine water treatment at Locality 1 plus the sulphate removal proposal
Actual cost of mine water treatment at Locality 2 plus the sulphates removal proposal.
To arrive at the figures in Table I, the total costs were split into fixed and variable costs so that the unit costs could be determined with regard to the capacity harmonization (i.e. the annual amount of treated mine water) of individual technological elements of the mine water treatment plants. The table shows that improved quality of the final effluent entails a significant increase in unit costs.
The higher treatment costs can be partially offset by using the mine's own water rather than purchasing water for technological purposes from a waterworks. Another option is to sell the treated mine water to other entities for technological or other purposes.
The analysis of the options for the respective Localities leads to the following results:
1. Use of mine's own water: the estimated amount of water purchased by a mining company is 200-300 thousand cubic meters per year. The use of the mine's own water instead would lead to savings worth tens of thousands of dollars.
2. Sale of the treated mine water: in principle, such water can be used for agricultural, industrial, and recreational purposes. As a rule, agricultural activity does not take place in the vicinity of opencast mines due to the impairment of the environment as a result of mining operations. Industrial companies already have their own water sources. Theoretically, artificial water bodies set up for recreational purposes may be an option, but these would involve substantial investments. Moreover, they would have to compete with natural water bodies in the region.
The yearly volume of treated mine water ranges from close to 1 million cubic meters at Locality 1 to 2 million cubic meters at Locality 2. The cost of mine water treatment is so high that it cannot be offset by the benefits stemming from the utilization of the treated mine water.
Conclusion
At present, the treated mine water from lignite coal mining is not used for commercial purposes in the Czech Republic. However, due to climatic changes or emergency situations, it may constitute an important prospective resource of surface water in the future. It has been proven that the quality of the final effluent from opencast lignite coal mining (after the existing technology is supplemented with the processes proposed) conforms to the requirements of a strategic water supply resource.
Acknowledgements
We would like to thank the Research Centre for Integrated System Development Concerning the Utilization of Byproduct of Energy Resource Mining and Processing for their support in the study.
References
1. CZECH REPUBLIC. Act No. 44/1988 Coll. on the Protection and Utilization of Mineral Resources (The Mining Act). www.cbusbs.cz/prehled-platnych.aspx Accessed 9 February 2010. [ Links ]
2. CZECH REPUBLIC. Act No. 254/2001 Coll. on Water and Amendments to Some Acts (The Water Act). Ministry of the Environment of the Czech Republic. www.mzp.cz/www/platnalegislativa.nsf/0/20f9c15060cad3aec1256ae30038d05c?OpenDocument&Click Accessed 25 January 2010. [ Links ]
3. CZECH REPUBLIC. Act No. 100/2001 Coll. on the Assessment of Impacts on the Environment and Amendments to Some Acts (Act on Environmental Impact Assessment). Ministry of the Environment of the Czech Republic www.mzp.cz/www/platnalegislativa.nsf/d79c09c54250df0dc1256e8900296e32/8a12b8f25817a234c125729d0039d956?OpenDocument Accessed 25 January 2010. [ Links ]
4. VIDLÁŘ, J., URBAN, V., and KINDIGER, G. Způsob čištění odpadních vod s nadlimitními obsahy síranů. Czech Pat. 290953: Appl. 6 March 1998, Acc. 13 November 2002. [ Links ]
5. VIDLÁŘ, J., NĚMEC, J., OSNER, Z., RACLAVSKÝ, J., SCHEJBAL, C., and STRNADOVÁ, N. Způsob úpravy důlních vod hlinitanem sodným. Czech Pat. 295200: Appl. 30 December 1998, Acc. 15 June 2005. [ Links ]
6. VIDLÁŘ, J. et al. Návrh technologického odstraňování síranů a rozpuštěných látek z důlníchvod lokality Kateřina-Radvanice (VUD). VŠB-TUO, červen 1998. [ Links ]
7. VIDLÁŘ, J. and HEVIÁNKOVÁ, S. Utility Model, CZ č.21442/2010: Donor of aluminium ions for sulphate precipitation. [ Links ]
8. BANNISTER, F.A., HEY, M.H., and BERNAL, J.D. Ettringite from Scawt Hill, Co. Antrim, Mineralogical Magazine, vol. 24, 1936. pp. 324-329. [ Links ]
9. HEVIÁNKOVÁ, S. and BESTOVÁ, I. Odstraňování manganu z kyselých důlních vod. Uhlí, Rudy, Geologickýprůzkum, vol. 15, no. 3, 2008. pp. 12-14. [ Links ]
Paper received Jul. 2010; revised paper received Oct. 2011.
© The Southern African Institute of Mining and Metallurgy, 2012. SA ISSN 0038-223X/3.00 + 0.00.














