Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 no.1 Johannesburg Jan. 2012
TECHNICAL NOTE
The plasma-assisted manufacture of zirconium metal powder from zirconium tetrachloride
J.T. Nel; J.L. Havenga; M.M. Makhofane; A.A. Jansen
The South African Nuclear Energy Corporation Ltd. (Necsa), South Africa
SYNOPSIS
The Kroll process is the traditional method of producing zirconium metal by reduction of zirconium tetrachloride with magnesium metal. This paper discusses the production of zirconium metal in a plasma reactor. Anhydrous low-hafnium ZrCl4 and Mg metal were fed continuously to a 30 kW non-transfer plasma reactor at a rate of 0.31 kg.h-1. Due to losses in the system the recovery of crude product was 77.5 %. After purification to remove unreacted feed material and MgCl2 by leaching, >98 % pure zirconium metal powder was obtained. The overall recovery of purified Zr was 74 %. The conversion efficiency of the process was 95 %.
Keywords: Zircon, zirconium, zirconium tetrachloride, plasma.
Introduction
South Africa is one of the world's largest suppliers of zircon sand, producing 30 % compared to Australian production of 36 % of the global demand of about 1 200 000 t in 20061. Zircon is used mainly as an opacifier in the ceramics industry, pigments, and also in the manufacture of various zirconium chemicals, stabilized zirconia ceramics, and zirconium metal1,2.
Zirconium finds wide application as fuel cladding material in nuclear power plants. In 2009 the demand for nuclear-grade zirconium metal was between 5 000 and 10 000 t. Production is closely related to nuclear energy demand3. Due to the close similarity of their chemical properties, zirconium and hafnium invariably occur together in nature. Commercial-grade zirconium metal contains up to 2 % hafnium However, hafnium has a thermal neutron absorption cross-section of 102 barns for 2 200 m.s-1 neutrons, while that of zirconium is 0.185 barns. Therefore nuclear-grade zirconium must be 'hafnium free' (< 100 ppm Hf)4. Zirconium is highly resistant to aqueous corrosion and radiation damage and has good mechanical properties even under intense radiation conditions4,5. Therefore it is a good choice for use in fuel-cladding material, e.g. in nuclear power reactors like pressurized water reactors (PWR's) and boiling water reactors (BWR's)6.
There are several methods available for the manufacture of zirconium metal, of which the Kroll process4,5,7-10 is the most widely used commercially. Zircon is generally used as precursor for the manufacture of zirconium tetrachloride feedstock for the Kroll process. The zircon (ZrSiO4) is carbochlorinated to produce zirconium tetrachloride (ZrCl4), silicon tetrachloride (SiCl4), and carbon monoxide (CO) according to Equation [1]:
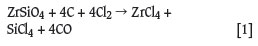
The relatively impure ZrCl4 separated from the SiCl4 during the carbochlorination process, still contains hafnium. In one of several methods to separate Hf and Zr4,5,11, hydrolysis of ZrCl4 is followed by liquid-liquid extraction as the oxychloride (ZrOCl2), followed by precipitation as Zr(OH)4 and thermal decomposition to ZrO2. The ZrO2 is then again carbochlorinated and the resulting ZrCl4 used as a feedstock in the Kroll process. In this process the ZrCl4 is reduced with magnesium metal in a sealed batch reactor at about 850ºC. Zirconium metal and magnesium chloride are formed in an exothermic reaction (Equation [2]).
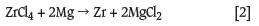
The magnesium chloride and any excess magnesium used in the reaction are removed from the reaction mixture by high-temperature vacuum distillation. The pyrophoric zirconium sponge so obtained is crushed, sorted, and purified by vacuum arc remelting to yield the metal as ingots. The metal can also be purified by the Van Arkel-De Boer process4,5,9,12-14, where it is allowed to react with a halogen (e.g. iodine) and the metal halide vapour is then decomposed on a white-hot (1 400ºC) tungsten wire to yield so-called crystal bar (Equations [3] and [4]):

This paper describes a continuous process in which ZrCl4 is reduced with magnesium in a plasma reactor to produce finely divided zirconium metal powder.
Equipment and process description
Equipment
The plasma system used for this work is schematically presented in Figure 1. A 30 kW direct current power supply provides power to a water-cooled non-transferred arc plasma torch. The plasma is started with argon and then switched over to run on nitrogen. Both the argon and nitrogen are >99.99 % pure.
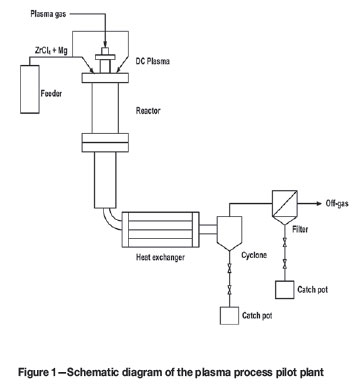
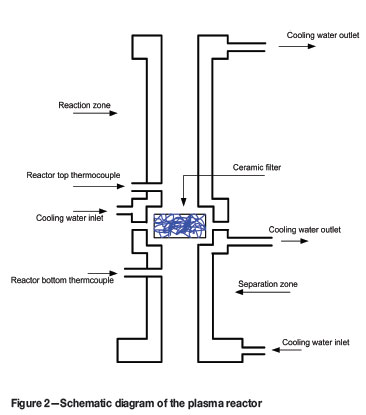
The reactor consists of a high-temperature reaction zone and a separation zone fitted with a ceramic filter (Figure 2). The reaction zone is 300 mm long and has an inside diameter of 40 mm. Both sections of the reactor are water-cooled. Gases and particulates passing through the ceramic filter then pass through a water-cooled elbow to a tube and shell heat exchanger, where they are finally cooled to near room temperature. The particles are removed from the off-gas stream by a cyclone and bag filter. The off-gas is scrubbed by 20 % (m/v) KOH solution before being released to the atmosphere.
Process description
An anhydrous mixture of ZrCl4 and a two times stoichiometric excess of Mg powder is entrained by a stream of dry nitrogen from a feeder mechanism and injected into the plasma tail flame. Both the ZrCl4 and Mg are vaporized by the plasma, which also provides the activation energy for the exothermic in-flight reduction reaction (ΔH = -854 kJ.kg-1 at 1 673 K (calculated by TerraTM software)). Physical and thermal properties of the reactants and products are presented in Table I.
The temperature measured in the separation zone of the reactor was about 1 400ºC (R-type thermocouple). At this temperature Zr is a solid, MgCl2 is close to its boiling point, and both Mg and ZrCl4 are in the vapour phase. Under these conditions one would expect to find only Zr and some MgCl2 on the filter. However, a mixture of Zr, MgCl2, Mg, and ZrCl4 was recovered from the ceramic filter and from the water cooled reactor walls. After the completion of a run, the system is cooled down to room temperature (about 10 minutes) and the deposits collected and stored under a dry nitrogen atmosphere.
Processing conditions
Cooling water flow rates and temperature differences are measured with calibrated instruments. Pressures reported here are gauge pressure (kPa(g))
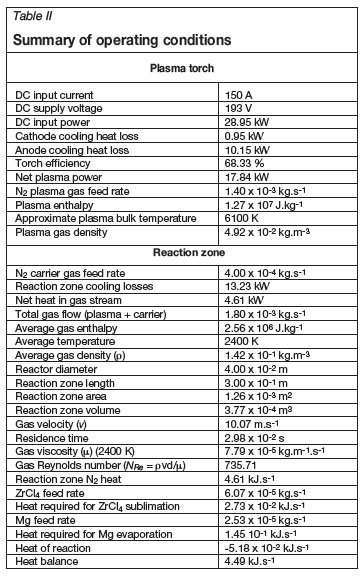
The energy balance and processing conditions are summarized in Table II. The heat balance was accurate to within 3.18 %. Enthalpy values for the plasma and reactor gas flow were calculated from the energy content and mass flow rates. The associated average temperature, viscosity, and density data were obtained from the standard work by Boulos, Fauchais and Pfender16. The average gas Reynolds number (NRe) in the reaction zone was well within the laminar flow regime.
Experimental procedure
Anhydrous ZrCl4 (purity >99.5 %; Hf < 50 mg.kg-1) was supplied by Sigma-Aldrich. Mg metal powder (>99 %) was supplied by Riedel-de-Haën. Since ZrCl4 is very hygroscopic it was stored under dry nitrogen. In this work a two times stoichiometric excess of Mg was used to ensure that adequate reductant was present in spite of possible incomplete mixing in the reaction zone. Stoichiometry and residence time were not optimized.
After starting up the plasma the reactor was allowed to warm up for 20 minutes at full power. The feeder, containing a pre-mixed anhydrous sample (59.47 g) of ZrCl4 and Mg, was suspended from a load cell so that the feed rate could be recorded. The feed system was started after warm-up of the reactor, the reagents entrained in dry nitrogen and injected into the plasma tail flame. A total of 51.6 g of the reagent mixture was consumed at a rate of 0.086 g.s-1 during a run lasting 11.5 minutes. A total of 40.0 g of crude product (77.5 % of the feed) could be recovered from the ceramic filter and the reactor wall. The 11.6 g of material unaccounted for is ascribed to inefficient recovery from the reactor walls and losses of vapour and finely-dispersed solids through the ceramic filter.
An aliquot of 30 g of crude product was purified by leaching with 65 % nitric acid at room temperature and the powdery residue washed with demineralized water for 10 minutes. The remaining solids were washed with ethanol, placed in a dessicator under a nitrogen atmosphere, and dried for 24 hours at 95ºC. The dried residue had a mass of 7.87 g. XRF analysis showed that the sample contained >98 % Zr.
It must be emphasised at this stage that this leaching procedure is not suitable for preparing nuclear-grade zirconium due to the risk of oxidation and the oxygen content of the metal being outside specification. However, for the purpose of this work, the wet leaching procedure is considered sufficient to illustrate that zirconium metal can be produced with reasonable purity in a plasma process.
Results
A photograph of the crude product is shown in Figure 3.

The product was analysed by scanning electron micropulse using energy-dispersive spectrometry (SEM-EPS), X-ray diffraction (XRD), and X-ray fluorescence (XRF), and combustion techniques.
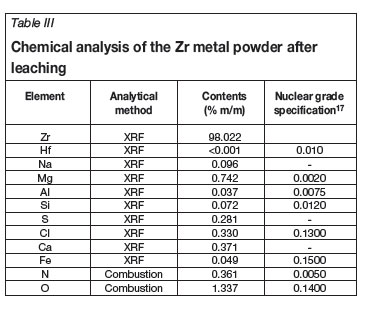
The chemical analysis of the Zr metal was conducted with XRF while a standard combustion technique was used to determine the oxygen (O) and nitrogen (N) contents. The results are presented in Table III. The presence of O in the samples can be attributed to an oxide layer formed on the metal during the leaching process and/or exposure to air. It might be possible to purify this product according to known methods like vacuum-arc melting. No hafnium was detected, since 'hafnium free' ZrCl4 was used as feed material. Figure 4 shows the scanning electron micrograph of the Zr powder at a magnification of about 1 500.

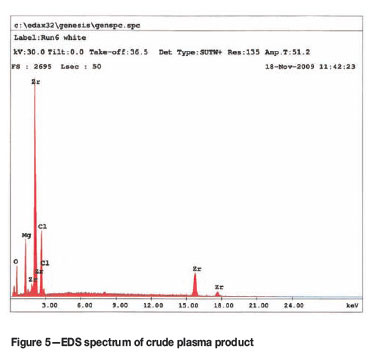
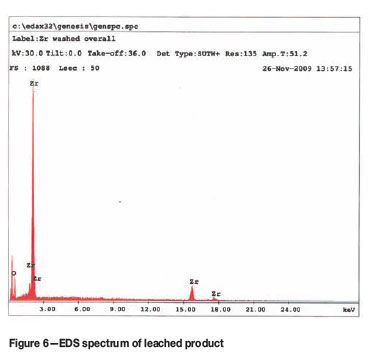
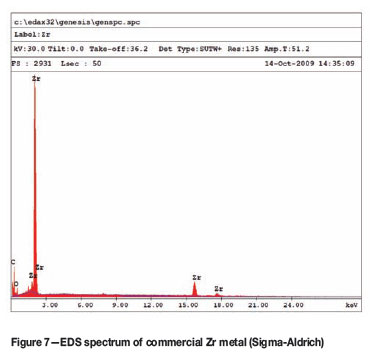
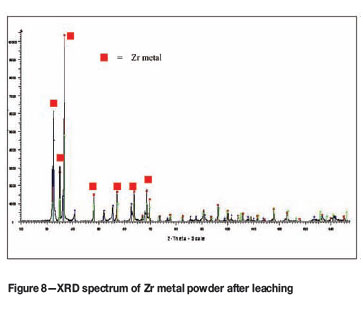
The SEM-EDS analysis of the crude sample (Figure 5) shows that the sample contained significant amounts of Mg and Cl as expected, which can be attributed to incomplete reaction. The SEM-EDS analysis of the leached sample (Figure 6) shows only Zr and traces of oxygen, confirming the effectiveness of the leaching procedure. This is in excellent agreement with the EDS spectrum obtained from a commercial sample of Zr metal obtained from Sigma-Aldrich (Figure 7). The XRD spectrum of the leached product (Figure 8) matches the Zr-metal spectrum of the 2007 PDF-2 database perfectly.
Discussion
A mass balance for the process is presented in Table IV. The product yield (Equation [5]) depends on conversion efficiency, reactant concentration in the gas phase, the time available for particle growth, filter efficiency, deposition onto cold surfaces, possible precipitation in the ceramic filter matrix, and nano sized particulates passing through the filter.
The conversion efficiency of the reduction process is influenced by factors such as particle loading, flow dynamics, uniformity of mixing and heating, possible stagnation zones, and residence time in the reaction zone. The calculated average gas enthalpy in the reaction zone corresponded to a temperature of 2 400 K for nitrogen obtained from standard reference tables16.
According to ideal stoichiometry (Equation [2]) the product mixture should contain Zr metal and MgCl2 although in practice the presence of excess Mg and some un-reacted ZrCl4 could be expected due to inefficient conversion.
The overall recovery (yield) of Zr metal was calculated as follows:
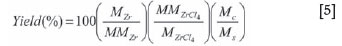
where MZr is the mass of Zr recovered after leaching, MZrCl4 is the mass of ZrCl4 fed to the process, MMZr and MMZrCl4 are the molecular masses of Zr and ZrCl4 respectively, Ms is the mass of sample subjected to leaching, and Mc is the mass of crude product recovered from the reactor.
The conversion efficiency, η, calculated according to Equation [6], was found to be 0.95.
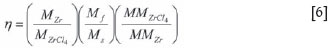
where Mf denotes the total mass of material fed to the reactor and the other symbols have the same meanings as before.
Vaporization of the solids in the plasma tail flame will depend on particle size and the heat and mass transfer rates, and is expected to be the overall rate-determining step. The heat and mass transfer rates for the reagents will be governed by the particle Reynolds numbers. According to Boulos, Pfender and Fauchais18 the particle Reynolds numbers under normal plasma conditions are < 100 and at these values increase linearly with particle diameter for spherical particles. Due to the complexities associated with modelling of such systems, and particularly, as in this case, for non-spherical particles, no attempt was made to estimate the particle residence time in the reaction zone. The gas residence time was calculated to be 29 ms. Owing to drag effects the average particle residence time is expected to be longer than this. Bourdin, Fauchais and Boulos19 calculated that it would take a 100 µm alumina particle between 10-3 and 10-2 seconds to reach melting point (2 326 K) in a 6 000 K nitrogen plasma. Metal particles, with their substantially higher thermal conductivity, took up to an order of magnitude less time to melt, so that one can expect that enough time was available for the Mg particles to melt, and by inference, for the ZrCl4 particles to sublime. However the molten Mg may not have vaporized completely in the time available and it is possible that the reaction could have been carried to completion on the filter surface. This would also explain the presence of both product and reactants in the crude product recovered from the filter.
Conclusions
Zirconium tetrachloride can be reduced by magnesium in an in-flight plasma process to produce zirconium powder of about 98 % purity after leaching of the crude product. The conversion efficiency of the process was 95 %. The overall Zr yield of about 74 % is attributable to incomplete conversion and losses of crude product during the high-temperature filtration and the subsequent product recovery process.
Acknowledgements
The contributions of the New Metals Development Network of the Advanced Metals Initiative of the Department of Science and Technology of South Africa for funding this project, and Necsa for the use of facilities and infrastructure, are highly appreciated.
References
1. ROSKILL. The Economics of Zirconium, 12th edn., London, Roskill Information Services Ltd, 2007. pp. 1,4. [ Links ]
2. SNYDERS, E. The Development of Zircon as a Superior Opacifier. D.Tech dissertation, Tshwane University of Technology, Pretoria, January 2007, p. 21. [ Links ]
3. http://nuclearstreet.com/blogs/nuclear_power_news/archive/2009/04/30/zirc.aspx. Accessed 4 Aug. 2010. [ Links ]
4. BENEDICT, M., PIGFORD, T.H., and LEVI, H.W. Nuclear Chemical Engineering, McGraw-Hill, USA,1981. pp. 318-347. [ Links ]
5. MILLER, G.L. Metallurgy of the Rarer Metals-2, Zirconium. Butterworths Scientific Publication, London, 1957. pp. 45, 79-106. [ Links ]
6. UX CONSULTING SERVICES. Nuclear Zirconium Alloy Market. April 2010. p. 11. [ Links ]
7. ABODISHISH, H.A.M. and KIMBALL, L.S. Process for purifying zirconium sponge, US Patent 5100465, 31 March 1992. [ Links ]
8. ISHIZUKA, H. Method for production of refractory metal from a chloride thereof, US Patent 4584018, 22 April 1986. [ Links ]
9. ELVERS, B. and HAWKINS, S. Ullmann's Encyclopedia of Industrial Chemistry, Zirconium and Zirconium Compounds, vol. A28, Fifth Completely Revised Edition. Wiley, New York, 1996, pp. 551-552, 558. [ Links ]
10. ISHIZUKA, H. Process for producing metallic zirconium, US Patent 4242136, 30 December 1980. [ Links ]
11. BARNES, D.E., BATCHELOR, R., MADDOCK, A.G., SMEDLEY, J.A., and TAYLOR, D. Newnes' Concise Encyclopaedia of Nuclear Energy. George Newnes Ltd, London, 1962. pp. 882-883. [ Links ]
12. SIDDALL, M.B. Iodide cell vapour pressure control. US Patent 4368072, 01 November 1983. [ Links ]
13. DE BOER, J.H. and VAN ARKEL, A.E. Process for precipitating hafnium and zirconium on an incandescent body. US Patent 1709781, 16 April 1929. [ Links ]
14. BLUMENTHAL, W.B. The Chemical Behavior of Zirconium. D. Van Nostrand Company, Inc., Princeton, New Jersey, 1958. p. 20. [ Links ]
15. CRC Handbook of Physics and Chemistry. 60th edn., CRC Press Inc, Boca Raton, Florida. [ Links ]
16. BOULOS, M.I., FAUCHAIS, P., and PFENDER, E. Thermal Plasmas Fundamentals and Applications, vol. I. Plenum Press, New York, 1994. [ Links ]
17. ASTM B349/B349M-09, Standard Specification for Zirconium Sponge and Other Forms of Virgin Metal for Nuclear Application, p. 223. [ Links ]
18. BOULOS, M.I., PFENDER, E., and FAUCHAIS, P. Plasma Technology in Metallurgical Processing. Feinman, J. (ed.), The American Iron and Steel Society, Inc, Warrendale, Pa, 1987, Ch. 5. [ Links ]
19. BOURDIN, I., FAUCHAIS, P., and Boulos, M.I. Transient heat conduction under plasma conditions, International Journal of Heat and Mass Transfer, vol. 26, no. 4, 1983. pp. 567-582. [ Links ]
Paper received Feb. 2011; revised paper received Jun. 2011.
© The Southern African Institute of Mining and Metallurgy, 2011. SA ISSN 0038-223X/3.00 + 0.00.














