Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 n.1 Johannesburg Jan. 2012
JOURNAL PAPER
Qualitative analysis of fine coals obtained from triboelectrostatic separation
S.O. BadaI, II; L.M. FalconI; R.M.S. FalconI; V.M. Du CannIII
ISchool of Chemical and Metallurgical Engineering, Faculty of Engineering and the Built Environment, University of the Witwatersrand, Johannesburg, South Africa
IIThe Mineral Processing Division, Mintek, Johannesburg, South Africa
IIIPetrographics SA, Pretoria, South Africa
SYNOPSIS
Coal samples taken from the No. 2 Seam and No. 4 Seam in a South African colliery were tested in a rotary triboelectrostatic separator. The two-stage triboelectrostatic separation results using the -177 µm size fraction reduced No. 2 Seam coal containing about 30.4 per cent ash to a clean product of 8.9 per cent and 13.1 per cent ash with combustible recoveries of 9.6 per cent and 30.7 per cent respectively. The same separation approach on No.4 seam feed coal, which contained 36 per cent ash, produced a clean coal product with 10.8 per cent ash at a combustible recovery of 6.0 per cent. Petrographic tests show a significant improvement in the vitrinite content and a reduction in visible minerals encountered when using the triboelectrostatic separator, with about 53 per cent vitrinite reporting to the clean fraction for No. 2 Seam compared to the feed content of 21 per cent, and a 54 per cent vitrinite in the clean No. 4 Seam from a feed content of 21 per cent. Significant sulphur reductions were also observed after separation in both coal samples, with a better separation in the second-stage coal products.
Keywords: coal, dry coal beneficiation, electrostatic separation, maceral, particle charging, RTS technique.
Introduction
Research into the triboelectrostatic separation of South African high ash coal was initiated in 2009 with a view to establishing whether fine, low-grade South African coals would respond to beneficiation using the principle of dry electrostatic separation. The motivation lay in the fact that South Africa has abundant lowgrade coal in need of beneficiation prior to use, and that this country is becoming increasingly short of water, a situation which may limit or preclude the use of large-scale wet beneficiation techniques in the medium to long term. Also, if proven technically and economically viable, it was hoped that the production of ultra-clean coals may lead either to high-grade fine products for use in pulverized fuel power stations and industrial boilers or to specialized ultra-low ash vitrinite-rich products for the manufacture of high-tech carbon materials such as carbon nanotubes1-3.
Currently, the coals utilized for domestic power generation in South Africa are unusually low in grade, typically with ash contents ranging from 29 per cent to 45 per cent and calorific values from 16 to 19.2 MJ/kg (Eskom4). Such low grades and qualities of coal are unlikely to meet the increasingly stringent environmental laws already enacted by different governments with specific reference to SOX, NOX, and particulate emissions5. High CO2 emissions may also arise due to the additional tons of coal required to produce a specific quantity of steam relative to that produced by a ton of low-ash high-grade coals.
Coal cleaning through the triboelectrostatic separation techniques could therefore offer both commercial and environmental benefits to producers and users of coal alike, by increasing the quality and commercial value of saleable coal. In so doing, the process has the potential to increase combustion efficiency and reduce maintenance in coal-fired plant and to enhance the environmental aspects of coal use through the production of low-sulphur and high-grade products which would, in turn, decrease the unburnt carbon loss during pulverized coal combustion.
In a previous study using a rotary triboelectrostatic separator on a range of South African coals, Bada et al.6 proved that fine organic particles of coal could be separated successfully from discreet mineral or rock particles. The level of efficiency or organic/ combustible recovery was found to be proportional to the degree of liberation between the organic matter and its associated mineral matter. The findings of these authors indicated that the finer the size, the greater the liberation, which leads, in turn, to greater degrees of combustible recovery and efficiency. With little or no adjustments to the operational features, the ash and sulphur contents in the primary product were reduced significantly thereby proving that considerably improved qualities of coal could be achieved. Further research to increase combustible recovery was proposed.
In principle, the triboelectric process separates the organic (maceral-rich) coal particles from mineral-rich particles through differential charges imparted to, and taken up by, the organic and mineral matter. These charges are enhanced by a rotary charger, which is held at a certain potential. Thus, a mineral particle would be charged negatively in the charging chamber and subjected to an attractive force from the positive electrode in the separation chamber, while the organic particle which is positively charged is subjected to an attraction from the negative electrode. Undertaking one pass of particles through the triboelectric chambers results in the production of three products; namely, a low-ash 'clean' coal product, a middling product and a mineral-rich 'discard' product. After passing through the chambers, these three products are all directed into different collecting bins and then analysed to determine the separation efficiency of the unit.
The current research was performed to determine the quality of coal and the yield of combustible matter attainable when applying triboelectric separation to samples from two commercially mined coal seams in South Africa, namely, No. 2 Seam and No. 4 Seam in the Witbank Coalfield. A series of tests was carried out to select optimum triboelectric separation parameters in order to obtain the maximum combustible recovery and highest product grades, and also to establish the effect of single- and two-stage separations. The parameters that were varied in the tests included different coflow velocity, feed rate, charger rotation speed, and splitter position. These parameters were applied to single- and twostage separation tests on all feed samples. Total sulphur, ash contents, and calorific values obtained from the single- and two-stage tests were analysed and compared, as well as the yields of the different separated products.
In addition to those analyses, the feed coals and the separated products were examined petrographically to determine the influence of triboelectric separation on the distribution of organic macerals between the separated products. Of the three main maceral groups, vitrinite was selected as the most important maceral to use for quality comparative purposes.
The results obtained are presented and discussed with regard to the potential application of the triboelectric separation technology to the beneficiation of South African fine coal.
Experimental techniques
Theory of triboelectrification
Tribocharging operates through both the contact charging and friction charging mechanisms by utilizing the differences in the surface electronic structure of the particles involved. In both cases, the mechanical processes that produce the charges on the materials are sliding, rolling or milling, impact and vibration of the surface at contact, and deformation leading to charge distribution at stress points7. In the case of the rotary charger that was used in this study, the particles were charged due to the collision between the cylindrical charging chamber, particle-particle contact, and the rotating charger octagonal copper plated surface. This action, known as triboelectrification, causes the transfer of negative charges (electrons) from the surface of one particle to another, with two different polarities within the contacted particles.
The magnitude of triboelectric charge exchanged between two contacting surfaces will depend on the differences in their electrical resistivities, their contacting relative speed, residence time, the difference in their work function, and on the pressure between the surfaces in contact. In addition, as the pressure increases, the area or the number of contact points increases, leading to a very high surface charge density.
The amount of energy used by the octagonal rotary charger for imparting differential surface charges on particles and using different tangential speeds was established in the investigation conducted by Bada7. The author hypothesized that triboelectrification occurs as a result of relative motion and exchange of charges between particles, with the imparted charges being maintained by the particles involved. Friction and transported gas within the separator were also considered to be responsible for the energy utilized by the octagonal rotary charger. The heat generated due to the action of the bearing friction increases the temperature of both the lubricant and neighbouring parts. The power used by the journal bearing, i.e. a simple bearing in which the shaft rotates in the bearing with a layer of grease separating the two parts, was established from the Spotts equation9.
Materials and test procedures
Two coal samples from No. 2 and 4 seams obtained from a colliery in the Witbank Coalfield, South Africa, were used for the triboelectrostatic separation experiments. The two samples provided coals of similar rank, characterized as Medium Rank Bituminous C. The coal samples were crushed in a hammer mill and then pulverized to -177 µm. A representative sample from each coal was prepared for triboelectrostatic separation. The coal samples were kept in an oven at 53ºC overnight to remove the surface moisture prior to separation.
For each test, 100 g of the representative sample was fed through a vibration feeder and transported pneumatically into the charging chamber, where the particles were imparted with different charges under variable voltage DC power supply and charger rotation speed of 2 500 r/min. Under the influence of the external electric field in the separation chamber, the positively charged particles were deflected towards the negative electrode (R plate), while the negatively charged particles were attracted towards the positive electrode (L plate), and the uncharged particles fell in-between the two electrodes and were collected as middling. The middling was then subjected to another stage of separation to generate three more products as shown in the flow sheet in Figure 1. The feed and products were analysed for ash content, total sulphur, calorific value, and petrographic composition. The performance curves generated from the ash recovery data were used to evaluate the separation performance of the No. 2 Seam and No. 4 Seam coals. The data reported in this publication was generated using results from single and two stage triboelectric separations.
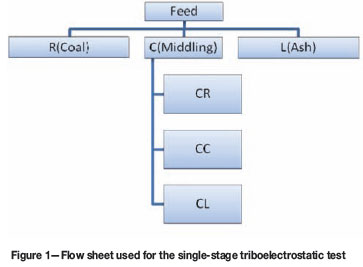
The schematic representation of the rotary triboelectrostatic separator used in this study has been reported elsewhere6. The experimental set-up included a vibratory sample feeder, rotary rotor mounted in a cylindrical chamber with a copper-plated surface for contacting and creating differential charges on particles, a chamber comprised of both an inlet for receiving the particles and an outlet for discharging the particles, a power source connected to the chamber's wall and the rotor, providing an electrical potential to the rotary charger. The unit was also made up of a separation zone consisting of two electrodes with a fixed distance between them, where charged particles from the charging chamber were deflected towards the negative or positive electrodes depending on the charge acquired. Lastly, an adjustable splitter was connected to three cyclones or airbags for the collection of the different products. The air flow within the separating compartment was monitored and regulated using a thermo-anemometer and gate valve connected to the nozzle under the collecting bin.
Results and discussion
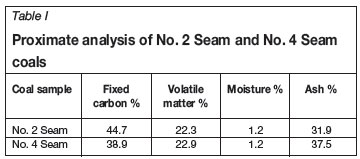
The proximate analyses obtained with -177 µm No. 2 and No. 4 seam coal samples are presented in Table I, which shows high ash and low moisture contents for both samples.
X-ray diffraction analysis
The diffractograms (Figure 2 for No. 2 Seam and Figure 3 for No. 4 Seam coal) show the X-ray diffraction patterns for the feed and product coal samples in the size ranges smaller than 177 µm. The intensity and height of the broad hump correspond to the quantity of organic matter present in the coal as organic material is amorphous10. It may be noted that the two feed coal samples show high peaks of crystalline minerals such as quartz, kaolinite, and pyrite and that there is a modest organic hump. The intensity of the quartz and kaolinite peaks reduces considerably in the cleaned products, with the two-stage product showing the least crystalline mineral presence.
Petrographic analysis
Petrographic analysis provides information regarding the organic (maceral) composition of a coal and the extent to which the macerals are intimately associated with minerals. The degree and nature of association between macerals and minerals are highly relevant as this information provides a measure of likely liberation between the organic and inorganic components in coal. It therefore has direct and significant application when assessing a coal for its beneficiation potential. For current purposes, both maceral and microlithotype analyses were undertaken. Of the three maceral groups in coal, vitrinite was selected as the most relevant organic component to describe for comparitive purposes due to its high value and desirable qualities. In terms of microlithotypes, several forms are important. Minerite represents fragments of rock or particles of extremely mineral-rich high-ash (dirty) coal. Carbominerite represents particles of coal with both organic and mineral matter intimately mixed, and vitrite represents particles of coal composed almost entirely of vitrinite. Other microlithotypes exist but are not relevant in the current situation.
Based upon the above description it will become obvious that the triboelectrostatic separator will be faced with separating a multitude of fine particles, some of which will be comprised of pure clean organic macerals (e.g. vitrite), some pure rocky fragments or mineral-rich dirty coal (minerite), and others will be mixed inorganic and organic forms (carbominerite). During early test work, it became apparent that particles charged positively in the charging chamber and collected from the negative plate electrodes showed a high vitrinite composition compared to the feed coal.
Tables II and III present the petrographic results conducted on the feed and the products obtained from the single- and two-stage triboelectrostatic separation tests. Vitrinite in No. 2 Seam increased significantly from 15 per cent in the feed coal to 53 per cent in the two-stage separation (Table II). Similarly, vitrinite in the No. 4 Seam increased from 21 per cent to 54 per cent. Minerite (rock particles) reduced in No 2 Seam microlithotypes from 19 percent to 2 per cent and in No 4 Seam from 19 per cent to 3 per cent (Table III). Carbominerite, i.e. particles with macerals and minerals intimately mixed together and which may be difficult to liberate from each other, reduced from 23 per cent in No. 2 Seam to 8 per cent in the two-stage process, and from 19 per cent to 8 per cent in No. 4 Seam.
These results indicate that the triboelectric separation process has clear and direct influence on the distribution of specific organic and inorganic components in coal, and that it is this phenomenon which gives rise to the changes in coal qualities as indicated by other forms of analyses (e.g. changes in ash or sulphur contents with separation). Furthermore, it is now apparent that separation takes place between suites of macerals as well as between minerals and macerals. This suggests that the triboelectrostatic process targets specific components within the maceral suites, and in this case, vitrinite in particular. Based upon the carbominerite content of the feed coals, it is also apparent that, with further reduction in particle size, minerals entrained in carbominerite particles could be liberated, thereby giving rise to even greater potential for particle separation and increase combustible recoveries.
Effect of splitter distance on single-stage separation
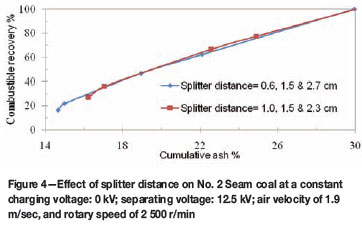
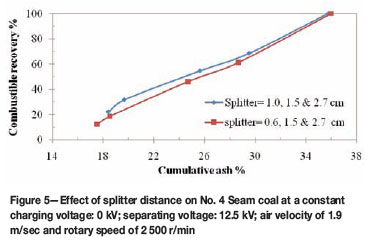
The position of the splitters is very important for the efficiency of the triboelectrostatic separator. It also influences the grade and recovery of the coal product. The product splitter position was adjusted to achieve different splits of clean coal products, middling, and tailings. For instance, when the splitter was tilted more towards the negative electrode, a cleaner coal product was collected at the expense of combustible recovery. No. 2 Seam and No. 4 Seam coals were subjected to triboelectrostatic separation at two different splitter set-ups, and the results are presented in Figures 4 and 5. The test was conducted under the single-stage separation process. Splitter positions were set at 0.6 cm and 1.0 cm away from the negative electrode. Cleaner coal of about 14.5 per cent ash and 17.4 per cent combustible matter was obtained when the splitter was moved 0.6 cm away from the negative electrode. In addition, 16.2 per cent ash with 28.1 per cent combustible matter was obtained when the splitter position was shifted 1.0 cm away from the negative electrode as shown in Figure 4. A similar trend is observed in Figure 5. The result shows that a cleaner coal product can be obtained if the splitter is positioned closer to the negative electrode.
Effect of charger rotation speed on two stage separation
The two-stage test results (Figures 6 and 7 for No. 2 Seam and 4 Seams) shows that the cleanest coal can be produced at 2 500 r/min as compared to other rotation speeds tested. For the two coals, the splitter was positioned at R=0.6 cm from the negative electrode, C at 1.5 cm and L at 2.7 cm from the positive electrode. The cleanest coal product of 8.9 per cent ash and 10.6 per cent ash were obtained at 2 500 r/min for No. 2 Seam and No. 4 Seam, respectively. A clean coal product with 16.3 per cent ash and 25.71 per cent yield was obtained at 4 000 r/min for No. 4 Seam coal. Higher recovery was observed at higher rotation speed for No. 4 Seam coal as compared to the result obtained for No. 2 Seam coal, which indicates virtually the same curve pattern. This may have been a result of particles reaching maximum charge density at this speed, thus a further increase in speed had little effect on separation or charging efficiency. Inculet11 stated that as the particle reaches its charge limit, charge backflow or discharging occurs. The lowest ash product was obtained at 2 500 r/min.
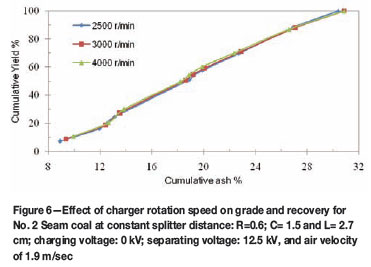
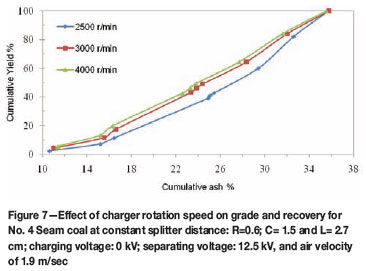
The cumulative yield versus cumulative ash curves presented in Figures 6 and 7 provide further evidence that, at 2 500 r/min rotation speed, a coal product with the lowest ash content can be obtained. An increase in rotation speed to 4 000 r/min does has no significant effect on the recovery of No. 2 Seam compared to that of No. 4 Seam. The increase in separation observed for No. 4 Seam coal may have been a result of increase in surface contact between the coal and the copper-plated surface, thereby increasing the particle charge density for No. 4 Seam coal.
Effect of co-flow
The separation efficiency for No. 2 Seam coal and No. 4 Seam coal was evaluated based on the grade and recovery of the coal products generated. The effect of co-flow rate was assessed in order to maximize coal cleaning. Co-flow, also known as flow straighteners, was used for sweeping away the particles drawn to the surface of electrodes and to reduce the turbulence flow by forming smooth co-flow of gas parallel to the feed stream upon entering the separation zone. The airflow within the separation zone was expected to be laminar so that the charged particles could be deflected only by the action of the electric field, which is of comparable strength to the drag force.
The co-flow test on No. 2 Seam coal was performed at a constant injection velocity of 1.9 m/sec, and a varied outlet velocity of 2.5 m/sec, 3.0 m/sec and 3.5 m/sec. The effect of co-flow speeds of 2.5 m/sec, 3.0 m/sec, and 3.5 m/sec for No. 2 Seam coal are presented in Figure 8. The highest grade product was obtained at the lowest co-flow velocity of 2.5 m/sec with a product ash of 8.8 per cent, and 9.8 per cent yield from a feed coal of about 30.5 per cent ash. At 3.5 m/sec co-flow velocity, another coal product with 9.4 per cent ash was also obtained for No. 2 Seam coal under the same two-stage test.
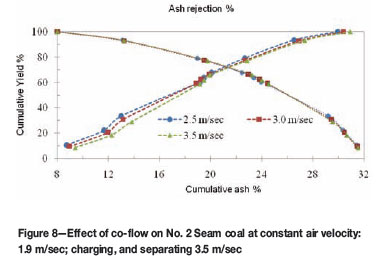
The coal quality attained at 2.5 m/sec outlet velocity may have been the result of a decrease in turbulent flow caused by the smooth stream of air within the separation zone as the outlet velocity decreases. Similar results were observed by Tao et al.12, where an increase in co-flow velocity unfavourably affected the separation capacity of the cylindrical rotary separator.
Effect of feed rate
The -177 µm coal samples were subjected to tribocharging in the octagonal rotary chamber at different feed rates. The results reveal that the particles respond differently to triboelectrostatic separation at different feed rates. The cumulative ash percentage versus cumulative yield percentage curves representing the effect of coal feed rate on grade and recoveries for No. 4 Seam coal are shown in Figure 9. These tests were conducted at 1.5 kV charging voltage, 12.5 kV separation voltage, 1.9 m/s injection velocity, 2 500 r/min rotary speed, and splitter distance of 0.6 cm from the negative electrode. A significant reduction in the ash content of the feed coal is observed as the feed rate increases from 12.8 g/min to 29.3 g/min, as shown in Figure 9.
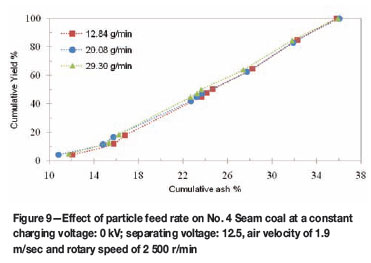
These results indicate that a feed coal of about 37 per cent ash can be reduced to 11.0 per cent ash product at 20.0 g/min, compared to a 12.2 per cent ash product obtained at 12.8 g/min for the same coal sample. Figure 9 also indicates that a cleaner coal product of 23.1 per cent ash and 48.2 per cent yield can be produced at a feed rate of 29.3 g/min. The increase in the particle concentration in the charging chamber (which generated more intensive particle/ particle collisions) might be responsible for an enhanced particle charging and better separation at the higher feed rate. These results are similar to those obtained by Tao et al.12 on carbon in fly ash removal using the rotary triboelectrostatic separator. Those authors found that the increase in yield and ash reduction is less sensitive to the feed rate in a rotary electrostatic separator, due to its efficient charging mechanism.
Summary of the calorific value and sulphur contents
The results of the calorific value and sulphur content analyses obtained for both single- and two-stage results are presented in Tables IV and V. The preliminary data obtained for both separation stages indicates that single-stage triboelectrostatic separation is effective in producing cleaner coals, but even cleaner products with higher grades and lower total sulphur contents are obtainable in the two-stage separation. These results, however, are currently counter balanced by lower combustible recoveries.
In Tables IV and V, it will be noted that No 2 Seam increased in calorific value from 20.8 MJ/kg to 26.9 and 29.4 MJ/kg in the one- and two-stage processes respectively, and No 4 Seam increased from 18.1 MJ/kg to 25.1 and 28.1 MJ/kg in the single- and two-stage processes respectively. The sulphur contents varied from 1.6 per cent in the No 2 Seam feed to 0.8 per cent and 0.7 per cent after single- and two-stage separation respectively, while No. 4 Seam varied from 1.6 per cent to 1.0 and 0.8 per cent respectively. The combustible recoveries varied from 6 per cent for No. 4 Seam to 30 per cent for No. 2 Seam.
Conclusions
This research study investigated the use of the rotary triboelectrostatic separation to upgrade fine coal from two commercial coal seams in South Africa. In so doing, it sought to determine the optimum process conditions for maximum ash reduction and product recovery, and to establish the factors responsible for the effective separation of mineral matter from macerals in the two coals under investigation. The conclusions are summarized as follows.
The rotary triboelectrostatic separation test results indicated that a good separation between organic matter and mineral matter can be achieved, leading to a considerable reduction in ash content after triboelectric separation and the production of a low ash coal product. Greater yields and lower ash values were achieved with the two-stage separation rather than the single stage test. Ash content for the No. 2 Seam coal was reduced from 30 per cent in the feed to 8 per cent and 13 per cent after two-stage and single stage separation, respectively, and from 36 per cent in the feed to 10 per cent in the cleaned product in No. 4 Seam coals after two-stage separation. Sulphur contents decreased from 1.6 per cent in the No. 2 Seam feed to 0.8 per cent and 0.7 per cent after single- and two-stage separation respectively, while No. 4 Seam varied from 1.6 per cent to 1.0 and 0.8 per cent respectively. Combustible recoveries increased from 6 per cent in No 4 Seam to 30 per cent in No 2 Seam
In terms of optimum operating parameters in the triboelectrostatic separator, the cleanest coal products were obtained when the splitter was positioned 0.6 cm away from the negative electrode compared with 1.0 cm away. The single-stage separation test on No. 2 Seam coal resulted in a 'clean' product with 14.5 per cent ash and 17.4 per cent combustible matter when the splitter was 0.6 cm from the negative electrode, and 15.7 per cent ash product with 28.1 per cent combustible matter when the splitter position was 1.0 cm away
The XRD analyses revealed that both feed coals contained high concentrations of quartz, kaolinite, and pyrite. These concentrations were shown to significantly and progressively reduce with single and twostage triboelectrostatic separation.
Petrographic analysis revealed a remarkable reduction in the visible mineral content and a marked increase in the organic maceral vitrinite for each sample tested, with the two-stage products having the highest vitrinite content. No. 2 Seam vitrinite increased from 15 per cent to 53 per cent in the two-stage separation, and No 4 Seam increased from 21 per cent to 54 per cent. Visible minerals (rock particles) reduced in No 2 Seam microlithotypes from 19 per cent to 2 per cent, and in No 4 Seam from 19 per cent to 3 per cent. Carbominerite, i.e. particles with macerals and minerals intimately mixed together (unliberated from each other), were also reduced from 23 per cent in No 2 Seam to 8 per cent in a two-stage process, and from 19 per cent to 8 per cent for No. 4 Seam
The triboelectrostatic separation process proved to be efficient in increasing the calorific value of the coals. Higher calorific coal products were obtained from the two-stage separation compared to that obtained from the single stage. No. 2 Seam increased in calorific value from 20.8 MJ/kg to 26.9 and 29.4 MJ/kg in the one-and two-stage processes respectively, and No. 4 Seam increased from 18.1 MJ/kg to 25.1 and 28.1 MJ/kg respectively.
In summary, despite the significant increases in coal qualities with triboelectric separation, relatively low combustible recoveries were obtained in these tests. It is anticipated, however, that with further reduction in particle size, which would result in increased liberation of mineral matter from organic matter, greater improvements both in combustible recovery and quality are likely to be achieved. This remains the subject for further study.
Acknowledgement
The authors gratefully acknowledge the financial support of the South African National Energy Research Institute (SANERI). Appreciation is also expressed to the Mineral Processing Division at Mintek and the Kentucky Geological Survey, for granting permission to perform XRD analyses.
References
1. YUA, J., LUCASA, J., STREZOVB, V., and WALLA, T. Coal and carbon nanotube production. Fuel, vol. 82, 2003. pp. 2025-2032. [ Links ]
2. TAIN, Y., ZHANG, Y., WANG, B., JI, W., ZHANG, Y., and XIE, K. Coal-derived carbon nanotubes by thermal plasma jet. Carbon, vol. 42, 2004. pp. 2597-2601. [ Links ]
3. ZHOU, Y., XIAO, N., QIU, J., SUN, Y., SUN, T., ZHAO, Z., and ZHANG, Y. Preparation of carbon microfibers from coal liquefaction residue, Fuel, vol. 87, 2008. pp. 3474-3476. [ Links ]
4. ESKOM. Lethabo Power Station General Fact Sheets. www.eskom.co.za/content/CO%200008PartiEmisContRev4~1.doc. 2007, Accessed April 2010. [ Links ]
5. CHIKKATUR, A.P. and CAMBRIDGE, M.A. Kennedy School of Government, Harvard University. Coal Initiative Reports: A Resource and Technology Assessment of Coal Utilization in India. 2008. [ Links ]
6. BADA, S.O., TAO, D., HONAKER, R.Q., FALCON, L.M., and FALCON, R.M.S.A. Study of rotary tribo-electrostatic separation of South African fine coal. International Journal of Coal Preparation and Utilization, vol. 30. no. 2, 2010. pp. 154-172. [ Links ]
7. MAZUMDER, M.K., SIMS, R.A., BIRIS, A.S., SRIRAMA, P.K., SAINI, D., YURTERI, C.U., TRIGWELL, S., DE, S., and SHARMA, R. Twenty-first century research needs in electrostatic processes applied to industry and medicine. Chemical Engineering Science, vol. 61, 2006. pp. 2192-2211. [ Links ]
8. BADA, S.O. Tribo-electrostatic separation of South African fine coal. PhD Thesis, University of the Witwatersrand. 2010. [ Links ]
9. SPOTTS, M.F. Design of Machine Elements. Prentice-Hall, Inc., Englewood Cliffs, New Jersey, 1985. 435 pp. [ Links ]
10. HUTTON, A.C. and MANDILE, A.J. Quantitative XRD measurement of mineral matter in Gondwana coals using the Rietveld method. Journal of African Earth Science, vol. 23, no. 1, 1996. pp 61-72. [ Links ]
11. INCULET, I.I. Electrostatic Mineral Separation. Research Study Press Ltd, Hertfordshire, UK, 1984. pp 153. [ Links ]
12. TAO, D., MAO-MING, F., and JIANG, X. Dry coal fly ash cleaning using rotary triboelectrostatic separator. Mining Science and Technology, vol. 19, 2009. pp. 0642-0647. [ Links ]
Paper received Sep. 2011; revised paper received Nov. 2011.
© The Southern African Institute of Mining and Metallurgy, 2011. SA ISSN 0038-223X/3.00 + 0.00.














