Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 no.1 Johannesburg Jan. 2012
JOURNAL PAPER
The preparation of activated carbon from South African coal
Q.P. CampbellI; J. R. BuntI; H. KasainiII; D.J. KrugerI
ISchool of Chemical and Minerals Engineering, North-West University
IISchool of Chemical Engineering, Tshwane University of Technology
SYNOPSIS
Activated carbons used in the precious metals extraction industry are characterized by large internal surface areas and a great affinity for metal ions. The purpose of this research is to prepare activated carbon from a South African bituminous coal by physical activation that is suitable and cost-effective for use in the extraction of metals. The quality of the coal-based activated carbon may not prove to be as good as activated carbon produced from other traditional sources, but the production costs involved may make South African coal a feasible alternative feedstock. The activated carbons produced were characterized by Brunauer, Emmett, and Teller (BET) surface area, activated carbon pH, and phenol adsorption studies and the results compared to the results from a commercially available activated carbon, Norit RO 0.8 (control sample). Bituminous coals from various sources including Witbank Seam 4 and Free State coal were used in this study. The preparation method chosen was physical activation using superheated steam. The effects of process variables such as activation time (1-3 h) and temperature (600-800ºC) were studied in order to optimize those parameters. The activated carbon surface area was characterized by means of nitrogen adsorption isotherms at 77K. BET surface area analysis showed that Witbank Seam 4 coal activated at a temperature of 800ºC and activation time of 3 hours resulted in a surface area of 340 m2/g. Quality control of each sample was performed by measuring the pH of a known amount of the prepared activated carbon in distilled water over time. Results showed that the pH of some of the prepared activated carbons reached a value of 11. Phenol adsorption results for the different activated carbons prepared corresponded well to the results obtained for the Norit RO 0.8 activated carbon sample.
Keywords: activated carbon, precious metal, South African coal, surface area, phenol adsorption.
Background and introduction
Activated carbon (also activated charcoal, activated coal), an amorphous, non-graphitic form of carbon, is characterized by a large specific surface area of 300-2 500 m2/g, which allows for the physical adsorption of gases and vapours as well as dissolved or dispersed substances from liquids1,2. Gas-phase carbons have predominantly fine pores, less than 100 Å in diameter; whereas the pore diameters for liquid-phase carbons have a broad range of 5-100 000 Å. The class of activated carbon produced depends primarily upon the raw material, and to a lesser extent upon the process of manufacture1. Activated carbon can be manufactured from any cellulosic or lignocellulosic material3. Bituminous coal is one of these types of materials that can be used to manufacture activated carbon because of its inherent microstructure4. Other precursors that have been proven successful are agricultural wastes, coconut shells5, pecan nut shells6,7, and even broiler manure8.
The activation of the raw material in order to prepare activated carbon can be carried out in two ways: chemical and physical activation9. Chemical activation is where the raw material is treated using any dehydrating agent that dissolves cellulosic components10,11. Physical activation is when the carbonaceous material is first devolatilized for an extended period of time (1-48 hours), and then the formed char is treated with any oxidizing agent such as carbon dioxide or steam. The oxidizing agent reacts with the carbon to form gaseous products, which results in pores and channels being created10,12.
Lorenc-Grabowska et al4 developed mesoporosity in lignite and gas coal (high-volatile bituminous coal) by coal modification using Ca- and Fe-exchange, and reported that a 20-25% increase of mesopore volume was observed. Gañan et al13 prepared high-porosity carbons from bituminous coal pitches by the combined use of chemical and physical activation. The activated carbons prepared using this process had surface areas in excess of 400 m2.g-1. Qiang et al14 investigated the selective adsorption properties of SO2 and NO onto coal-based activated carbon. Hsu and Teng15 prepared activated carbon from bituminous coal via chemical activation (ZnCl2, H3PO4, and KOH), after which the sample was carbonized in nitrogen at various temperatures. It was found that ZnCl2 and H3PO4 were not suitable for preparing high-porosity carbons from bituminous coal, while KOH can produce carbons with high porosity. Mineral matter content can affect the adsorptive capacity of an activated carbon. Linares-Solano et al16 investigated this subject and reported that the samples with lower ash content yielded higher micropore volumes.
Numerous articles have also been published with regards to the extraction of metal utilizing activated carbon. Riaz Qadeer17 has performed extensive research on the adsorption of ruthenium ions on activated carbon. The removal of this ion is important for purification, waste treatment, and trace metal analysis. Yalcin and Ihsan Arol18 reported that due to the expansion of the gold industry, the use of coconut shells as raw material for the production of activated carbon suitable for gold adsorption from cyanide leach liquors is costly and the exploitation of other raw materials should be considered. They investigated the use of hazelnut shells, apricot and peach stones, and studied the gold loading capacity and adsorption kinetics. Zhang et al19 studied the adsorption of the gold thiourea complex onto activated carbon and found that equilibrium gold loading decreased with an increase in thiourea concentration, solution pH, and temperature. Fraga et al20 investigated the role of carbon surface sites on the properties of carbon-supported platinum catalysts. They pre-treated a commercially available activated carbon (Norit ROW 0.8 s) and performed oxidative treatments on the carbon to create surface acidic sites and destroy surface basic sites. Fu et al21 studied the adsorption and reduction of Pt(IV) on activated carbon fibre (ACF). They investigated the relationship between the adsorption reduction of the metal and the activated carbon preparation conditions, as well as adsorption conditions (solid/liquid ratio, Pt(IV) concentration, reaction temperature, and solution pH). They concluded that Pt(IV) adsorption-reduction capacity onto normal activated carbon fibres is not proportional to the specific surface area. Jia et al22 investigated the adsorption characteristics of gold and silver cyanide anionic species on a suite of activated carbons derived from coal, coconut shell, and polyacrylonitrile. Kasaini et al23 investigated the selective adsorption of platinum from mixed chloride solutions containing base metals using chemically modified activated carbons.
From the above it is clear that there are many parameters that affect carbon performance. The amount and distribution of pores play key roles in determining how well contaminants are adsorbed. Adsorption is also affected by the chemical nature of the adsorbing surface because the surface interacts to a certain extent with organic molecules. Electrical forces between the surface and some organic molecules may also result in adsorption or ion exchange. But generally, the least soluble organic molecules are most strongly adsorbed, and often the smaller organic molecules are held the tightest, because they fit into the smaller pores. Adsorption usually increases as pH and temperature decrease and contact time is increased.
In South Africa, current precious meta.l concentrator plants and refineries are unable to retrieve all of the valuables, and significant quantities of precious metals are discarded on tailing dams. The scope of the project is to produce an activated carbon that will be able to adsorb precious metal ions from dilute solutions. Other studies24 have proven that Norit RO 0.8 is suitable for this adsorption process, but this material has to be imported. It has been shown that South African coal can be used as raw material for the manufacturing of activated carbon25, but studies have not been conducted to explore further applications of the activated carbon thus produced. This study has therefore aimed to produce an activated carbon, using South African coal, which must meet certain criteria specifically concerning surface area and adsorption characteristics. The successful activated carbon will be used in later adsorption studies to characterize the success of the choice of raw material and preparation method, which will form the basis of a future paper. Two South African coals were selected as precursor materials for the preparation of activated carbon. Experiments were conducted to determine the optimum parameters such as activation time and temperature. BET surface area analysis, pH measurements, and phenol adsorption were used to quantify whether the prepared activated carbon is of a suitable quality. Key characteristics that influence the suitability of activated carbon for use in a production plant i.e. hardness (durability), particle shape (important for screening), and potential for fouling by organics or other contaminants falls outside the scope of this study and are thus not considered.here. The intention of this article is not to demonstrate kinetics and equilibrium loadings of PGM or Au from solution using the coal-derived active carbons, but rather to use indirect indicators such as Brunauer, Emmett, and Teller (BET) surface area and phenol adsorption to indicate potential for metal loading.
Experimental
To achieve the desired results in manufacturing an activated carbon meeting all criteria, an experimental process needed to be considered. This section considers the experimental procedure that was followed, and addresses the equipment that was used as well as the characterization methods employed. It should be noted that the precious metal application testing of the coal-derived active carbons is excluded from this exploratory study. General process considerations, i.e. loaded metal ions adsorbed from the leach solution using agitated tanks with interstage screens followed by elution, were used to determine which properties are important. This philosophy assisted in determining the performance criteria chosen to measure (BET surface area, phenol adsorption, and pH) which are indeed the most important parameters for the application. Abrasion and screening testing of the loaded carbon should be considered in future adsorption studies.
Coal
The raw coals used in this study were sourced from two areas in South Africa, i.e. Witbank seam 4 (Kriel - opencast), and Free State coal (New Vaal Colliery). Both of these coals are reasonably representative of coal in each of these areas as can be seen from the proximate analysis results (Table I) compared with the literature26. The as-received (a.r.) samples were stage crushed and sieved to a narrow particle size range (+860 µm -1 180 µm), cleaned using a dilute solution of 1M HCl to remove any inorganic material, and then washed using distilled water, filtered, and dried under vacuum at 105ºC for approximately 6 hours. Proximate analysis was conducted on the sized coal samples.
Activation reactor setup
The main components in the experimental setup (as shown in Figure 1) include a vertical tube furnace capable of reaching temperatures of up to a 1 000ºC, and a steam generator with a piston pump delivering the required flow rate of steam.
The water for the steam generator was supplied via a reservoir which was located on a load cell. The mass change (W) was recorded, from which the steam flow rate was determined. It was assumed that the mass loss in the reservoir was an indication of the steam flow rate supplied to the furnace. The steam generator was controlled at a temperature of 160ºC. The steam was mixed with nitrogen and passed through a heating stage which was also controlled at 160ºC. The piping to the furnace was well insulated to ensure minimal heat loss. The steam/nitrogen mixture was then passed over the sample in the furnace. A thermocouple in the reactor ensured that the sample temperature was monitored and logged.
The length of the reactor was 200 mm, while the length of the heating zone was 300 mm. This ensured that both the coil and the reaction zone were at the controlled temperature. The coil ensured that the steam/nitrogen mixture was brought from its initial temperature of ±160ºC to the temperature that the furnace was controlled at.
With each experimental run, 20 gr of the sized and cleaned coal was firstly devolatilized using nitrogen prior to steam activation. This sample amount occupied approximately half of the volume of the reactor. The devolatilization temperature was varied between 600, 700, and 800ºC; and the steam activation experiments, using superheated steam at a flow rate of 13.7 cm3/(g.hr) was varied for periods of 1, 2, and 3 hours. After reaction, the furnace was allowed to cool to below 45ºC before removal of the sample.
Activated carbon characterization
The prepared activated carbon samples were characterized using BET surface area determination, phenol adsorption studies, and carbon pH testing. These results were compared to the commercially available activated carbon (Norit RO 0.8). The NORIT reference carbon was chosen as a benchmark based on similarity of application; i.e. peat-derived, high surface area.
BET surface area
The surface areas of the coal-derived activated carbons were determined by means of nitrogen adsorption experiments in a Micrometrics ASAP 2010 Analyzer. Initially the samples were degassed at 25ºC for a period of 48 hours. After degassing, the carbons were analyzed by means of gas adsorption using nitrogen as sorbent. All of the gas adsorption experiments were done at a temperature of 77K using a saturation pressure (P0) of < 660 mm Hg.
Phenol adsorption studies
Activated carbon was stirred in a phenol solution and samples were taken at various intervals. These samples were then analyzed using a ultraviolet spectrophotometer (UV-vis) to determine the phenol concentration of the parent solution. The concentration results reflect the amount of phenol that was adsorbed by the activated carbon. Before the phenol concentration could be determined, it was necessary to obtain a calibration curve. This was performed by measuring the adsorbance of different concentrations of phenol with the use of the UV-vis. The adsorbance data was plotted against the different concentrations and a straight line was fitted.
pH measurement
Two grams of the activated carbon sample was added to 40 cm3 of distilled water and stirred at a speed of 1 100 r/min. The pH of the solution was measured every 5 minutes for a period of 200 minutes. When the activated carbon and de-ionized water was mixed and stirred, it was observed that the maximum pH value was reached within 60 minutes. It was also seen that after reaching the maximum pH value for a specific sample, the pH gradually decreased and did not stabilize.
From the measured pH results, the following calculations were applied and graphs were obtained for the different samples.
Δ pH versus time(s)

Δ pH /dt versus time(s)

pH versus time(s) to obtain adsorption constant *
pH versus time(s) to obtain de-sorption constant *.
The adsorption and desorption rates were determined from the initial and final slope of the pH versus contact time graphs. All tests were conducted in duplicate to assess repeatability, and the averaged data is presented in the discussion of the results.
Results and discussion
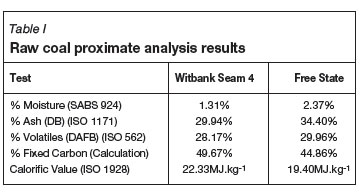
The proximate analysis results for the Witbank 4 seam coal and the Free State coal are given in Table I. These will be discussed prior to reviewing the activated carbon characterization results.
From Table I it can be observed that the feed coal moisture contents were 1.3% and 2.4% for the Witbank seam 4 and Free State coals respectively. On a dry basis, the respective ash contents were 30% and 34.4%; and on a dry ash free (d.a.f.) basis the volatile matter contents were reasonably similar, i.e. 28% and 30%. The fixed carbon percentage was calculated to be higher for the Witbank Seam 4 coal (49.7%) when compared to the Free State coal (44.9%). Thus the heating value (ISO 1928) was also measured to be higher for the Witbank seam 4 coal (22.3 MJ/kg) compared with 19.4 MJ/kg for the Free State coal. These results compare well with the data supplied by the Department of Minerals and Energy for these two raw coals27.
BET surface area results
The surface area results for the activated carbons prepared from both the Witbank Seam 4 coal and the Free State coal are graphically depicted in Figure 2. Before experimentation commenced on the Free State raw coal, it was decided to assess the final activated carbon product only at an activation time of 2 and 3 hours, since the results from the Witbank Seam 4 raw material showed that very low surface areas were obtained at an activation time of 1 hour (4-10 m2/g).
It can be observed from Figure 2 that the surface areas for the Witbank seam 4 carbons generally increased with increasing activation temperature and activation time. At a temperature of 600ºC the surface area was 4.7m2/g after 1 hour of activation. After 2 hours of activation (at 600ºC) the surface area increased to 41.7 m2/g, and finally to 176 m2/g after 3 hours of activation. When the temperature was increased to 800ºC, the surface area increased from 9.8 m2/g after 1 hour of activation to 159.4 m2/g and 338.3 m2/g after 2 and 3 hours of activation time respectively. This increase in the surface area is also an indication of the quantity of adsorption sites that are created with increasing activation time and temperature. The effect of the large quantity of adsorption sites will be discussed with reference to phenol adsorption in the following section.
The surface area measurements obtained for the activated carbon samples prepared from Free State coal exhibit the same trend as the Witbank seam 4 activated carbon samples, i.e. an increase in the overall surface area is observed with an increase in activation time and temperature. At a temperature of 600ºC and an activation time of 2 hours, the surface area was 44 m2/g for the Free State carbon. However, at higher temperature (800ºC) and activation time (3 hours), the Free State activated carbon surface area decreased from 190 m2/g (2 hours) to 158.1 m2/g, this decrease was not observed in the case of the Witbank Seam 4 activated carbons.
Previous studies28-30 on the influence of minerals in coal on gasification and reactivity have shown that a higher mineral content increases the average density, and decreases the available surface area and porosity of the coal particles. During the heating of coal, vitrinites and all other reactive macerals soften, and after a while degasify. The release of gasses creates pores, and as these gases increase in volume, the walls soften and expand. This causes the material to increase in volume and surface area. The decrease in surface area at higher temperatures and activation time for the Free State carbon can possibly be explained by the fact that the higher density coals (5% higher ash content than for the Witbank seam 4 coal) tend to form thick-walled dense chars26-28 with low surface area.
The standard Norit RO 0.8 activated carbon was found to have a surface area of 757.8 m2/g. It is clear that the highest surface areas obtained for the activated carbons prepared from Witbank Seam 4 coal (338 m2/g) and for the Free State coal (181.7 m2/g) do not meet the surface area requirement when compared to that of Norit RO 0.8.
Phenol adsorption results
Figure 3 shows the initial phenol adsorption results (ppm/min) which were obtained for the different activated carbon samples prepared from the Witbank Seam 4 raw coal. In these results the initial phenol adsorption rate increased with activation temperature and activation time. Furthermore, the adsorption rate increased from 4.7 to 8.9 ppm/min for carbons prepared at 600ºC and 800ºC and an activation time of 1 hour. When the activation time is further increased to 2 hours, the adsorption rate increased from 4.4 to 17.2 ppm/min within the same temperature range. After 3 hours of activation, the adsorption rate increased even further, i.e. from 5.6 to 22 ppm/min at temperatures of 600ºC and 800ºC respectively.
Figure 4 shows the phenol adsorption results obtained for the different activated carbon samples prepared from Free State coal. The rate at which the Free State activated carbon adsorbed phenol (20.5 ppm/min) was similar to that of Witbank seam 4 activated carbon (22.0 ppm/min). When the activation temperature and time are increased, the phenol adsorption rate increases up to the point where maximum loading occurs.
There is thus a relationship between the BET surface area (Figure 2) and phenol adsorption rate (Figures 3-4), i.e. a higher surface area results in a higher phenol adsorption rate. When the phenol adsorption rates for Witbank seam 4 activated carbon are compared to Norit RO 0.8 (27.8 ppm/min), the Witbank seam 4 activated carbon sample, (prepared at 800ºC and 3 hours) showed the best resemblance (22.0 ppm/min). Desorption for the respective samples was not evident, since phenol loading onto the total carbon surface occurred to the extent where all adsorption sites are occupied.
Activated carbon pH. results
Figures 5 and 6 show the measured pH values obtained for the Witbank seam 4 carbon and Free State carbon samples respectively. All the coal-derived carbons changed the nature of the water to basic during the pH analysis. Faust and Aly31 found that when an H-type activated carbon is produced (activated at temperatures above 650ºC and exposed to steam or CO2), it is hydrophobic in nature and tends to take on a positive charge by adsorbing the H+-ions when immersed in water (most of the surface oxides present are basic in nature). On the other hand, the L-type carbon absorbs the hydroxyl (OH-) ions when immersed in water and the surface oxides formed are acidic in nature. Thus, the H-type carbon behaviour can clearly be seen for the activated carbons that were prepared from both the Witbank Seam 4 and Free State raw material, as well as for the standard Norit RO 0.8 activated carbon.
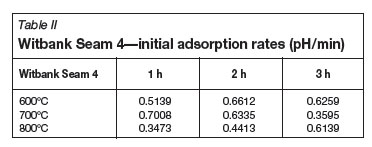
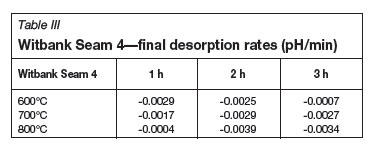
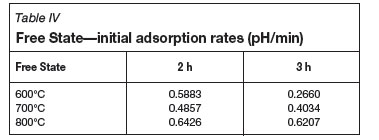
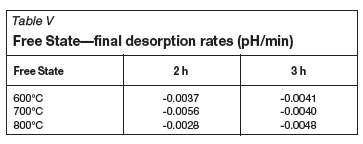
Tables II-V show the calculated initial adsorption and final desorption rates for all of the coal-derived activated carbon samples. Considering the pH results obtained for the Norit RO 0.8 control samples (initial adsorption rate of 0.802, and desorption rate of -0.0003), the aim is to have a high H+ adsorption rate, and thereafter a constant activated carbon/water pH within the given time limit. It is noted that there are no fixed relationships between the measured pH results and the activation temperature and time. It can also be observed that the pH rate results of the Free State activated carbon and Witbank seam 4 activated carbons are similar with regards to the initial adsorption rates and final desorption rates.
Concluding remarks
During this investigation, activated carbon was prepared from two different types of South African coal. An activation method was chosen and activated carbon samples were prepared at different temperatures and activation periods. The activated carbons were characterized according to BET surface area, pH, and phenol adsorption, the results compared to the Norit RO 0.8 control sample. Although the surface area measurements did not compare well with that of the control sample, pH and phenol adsorption studies of the prepared activated carbons proved that they exhibit the same adsorption characteristics.
The BET surface area results that were obtained for the activated carbon samples prepared from the Witbank Seam 4 coal samples, prepared at the lower activation temperature (600ºC) and activation time (1 hour) indicated very low surface areas (approximately 5 m2/g). Samples prepared at higher temperatures (800ºC) and longer activation times (3 hours) had higher surface areas (approximate 340 m2/g). Therefore it can be concluded that for the Witbank Seam 4 coal, a higher activation time and temperature will result in activated carbon product having a higher surface area. The highest surface area that could be obtained for the Free State raw coal was the sample activated at 800ºC and 2 hours, which resulted in a BET surface area of approximately 190 m2/g. The conclusion regarding the effect of activation time and temperature is thus not similar to the case of the Witbank Seam 4 activated carbon, where high activation temperatures and activation time deliver a sample with a high BET surface area. When the above results are compared with surface area results obtained for the Norit RO 0.8 control sample (757 m2/g), it can be seen that only the activated carbon prepared from the Witbank Seam 4 raw material corresponded well.
From the results obtained from phenol adsorption studies for the Witbank Seam 4 activated carbon, it was found that there is a strong relationship between the extent to which the sample was activated (activation time and temperature) and phenol adsorption characteristics. With increasing activation time and temperature, an increase in the amount of phenol adsorbed is apparent. Similar adsorption results were obtained when the Witbank Seam 4 activated carbon adsorption results were compared to those obtained for the Norit RO 0.8 activated carbon. The initial adsorption results for the activated carbon prepared at 800ºC for 3 hours showed the closest resemblance to the adsorption characteristics of the Norit RO 0.8 activated carbon.
The relationship between BET surface area and phenol adsorption can also be seen in the activated carbons prepared from the Free State coal. With increasing activation time and temperature, an increase in the amount of phenol adsorbed is observed. When these results were compared to the adsorption results from the Norit RO 0.8 activated carbon, similar trends are observed. The activated carbon prepared at 800ºC and 2 hours had the highest adsorption rate. This specific sample also had on average a higher BET surface area compared to the other Free State activated carbon samples.
From the pH measurements it can be concluded that both the Witbank seam 4 raw material and the Free State raw coal yielded an H-type activated carbon which corresponds well with what the literature predicts. The Witbank Seam 4 activated carbon pH compared well with the control carbon, but for the Free State carbon, the value decreased after the maximum solution pH had been reached.
Further studies should focus on manufacturing activated carbon from South African coal, applying it for adsorption, and exploring ways in which the activated carbon can be successfully reactivated for repeated use in an adsorption process. South African coal also inherently contains a large amount of mineral matter. It can be seen that this figure is as high as 35% (from the proximate analysis of Free State coal). Methods should be explored to determine if a reduction in ash content of South African coal can deliver an activated carbon with enhanced adsorption properties compared to those of a coal without ash reduction. A Fourier transform infrared spectroscopy (FTIR) analysis of the activated carbon sample can determine the functional groups on the surface of the carbon. This should be incorporated into future studies to help to better explain the adsorption behaviour of the activated carbon as shown by pH measurements and phenol adsorption studies. Key characteristics that influence the suitability of activated carbon for use in a production plant i.e. hardness (durability), particle shape (important for screening), and potential for fouling by organics should also be considered in future studies.
References
1. SNELL, F., HILTON, C. and ETTRE, L. Encyclopedia of Industrial Chemical Analysis, vol. 8. InterScience, New York, 1974. 738 pp. [ Links ]
2. KIRK, R. and OTHMER, D. Encyclopedia of chemical technology, vol. 4. InterScience, New York, 1956. [ Links ]
3. ALMANSA, C., MOLINA-SABIO, M., and RODRIGUEZ-REINOSO, F. Adsorption of methane into ZnCl2-activated carbon derived discs. Microporous and Mesoporous Materials, vol. 76, 2004. pp. 185-191. [ Links ]
4. LORENC-GRABOWSKA, E., GRYGLEWICZ, G., and GRYGLEWICZ, S. Development of mesoporosity in activated carbons via coal modification using Ca- and Fe-exchange. Microporous and Mesoporous Materials, vol. 76, 2004. pp. 193-201. [ Links ]
5. BANDOSZ, T. Effect of pore structure and surface chemistry of virgin activated carbons on removal of hydrogen sulphide. Carbon, vol. 37, 1999. pp. 483-491. [ Links ]
6. DASTGHEIB, S. and ROCKSTRAW, D. Pecan shell activated carbon: synthesis, characterization and application of for the removal of copper from aqueous solution. Carbon, vol. 39, 2001. pp. 1849-1855. [ Links ]
7. DASTGHEIB, S. and ROCKSTRAW, D. A model for the adsorption of single metal ion solutes in aqueous solution onto activated carbon produced from pecan shells. Carbon, vol. 40, 2002a. pp. 1843-1851. [ Links ]
8. LIMA, I. and MARSHALL, W. Granular activated carbons from broiler manure: physical, chemical and adsorptive properties. Bioresource Technology, vol. 96, 2005. pp. 699-706. [ Links ]
9. CAMERON CARBON. www.cameroncarbon.com. Accessed 9 October 2007. [ Links ]
10. MCKETTA, J.J. Encyclopaedia of chemical Processing and design, vol. 1. Dekker, New York, 1976. 452 pp. [ Links ]
11. SMISEK, M. and CERNY, S. Active carbon: manufacture, properties and applications. Elsevier, New York, 1970. pp. 10-48. [ Links ]
12. HABASHI, F. Principles of Extractive Metallurgy, vol. 2. Gordon and Breach, New York, 1986. 390 pp. [ Links ]
13. GAÑAN, J., GONZÁLEZ-GARCÍA, C.M., GONZÁLEZA, J.F., SABIO, E., MACÍAS-GARCÍA, A., and DÍAZ-DÍEZ, M.A. Preparation of activated carbon from bituminous coal pitches. Applied Surface Science, vol. 238, 2004. pp. 347-354. [ Links ]
14. QIANG, T., ZHIGANG, Z., WENPEI, C., and ZIDONG, Z. SO2 and nonselective adsorption properties of coal-based activated carbons. Fuel, vol. 84, 2005. pp. 461-465. [ Links ]
15. HSU, L. and TENG, H. Influence of different chemical reagents on the preparation of activated carbons from bituminous coal. Fuel Processing Technology, vol. 64, 2000. pp. 155-166. [ Links ]
16. LINARES-SOLANO, A., MARTÍN-GULLON, I., SALINAS-MARTÍNEZ DELECEA, S., and SERRANO-TALAVERA, B. Activated carbons from bituminous coal: effect of mineral matter content. Fuel, vol. 79, 2000. pp. 635-643. [ Links ]
17. QADEER, R. Adsorption of ruthenium ions on activated charcoal: influence of temperature on the kinetics of the adsorption process, Journal of Zhejiang University, vol. 6B, no. 5, 2006. pp. 353-356. [ Links ]
18. YALCIN, M. and IHSAN AROL, A. Gold cyanide adsorption characteristics of activated carbon of non-coconut shells origin. Hydrometallurgy, vol. 63, 2002. pp. 201-206. [ Links ]
19. ZHANG, H., RITCHIE, I.M. and LA BROOY, S.R. The adsorption of gold thiourea complex onto activated carbon. Hydrometallurgy, vol. 72, 2004a. pp. 291-301. [ Links ]
20. FRAGA, M.A., JORDÃO, E., MENDES, M.J., FREITAS, M.M.A., FARIA, J.L., and Figueiredo, J.L. Properties of carbon-supported platinum catalysts: role of carbon surface sites. Journal of Catalysis, vol. 209, 2002. pp. 355-364. [ Links ]
21. FU, R., LU, Y., XIE, W., and ZENG, H. The adsorption and reduction of Pt(IV) on activated carbon fibre. Carbon, vol. 36, nos. 1-2, 1998. pp. 19-23. [ Links ]
22. JIA, Y.F., STEELE, C.J., HAYWARD, I.P., and THOMAS, K.M. Mechanism of adsorption of gold and silver species on activated carbons. Carbon, vol. 36, no. 9, 1998. pp. 1299-1308. [ Links ]
23. KASAINI, H., EVERSON, R., and BRUINSMA, O. Selective adsorption of platinum from mixed solutions containing base metals using chemically modified activated carbons. Separation Science and Technology, vol. 40, 2005. pp. 1-17. [ Links ]
24. MBAYA, R.K.K. and Kasaini, H. Separation of platinum chlorocomplex ions from base metal ions in fixed bed columns containing activated carbons. Precious Metals 07, Brisbane, Australia, 2007. [ Links ]
25. WELMAN, B. Die bereiding van geaktiveerde koolstof uit steenkool. M.Sc. thesis, School for Chemistry, North-West University. 1990. [ Links ]
27. DEPARTMENT OF MINERALS AND ENERGY. Annual Report on Coal Sources in South Africa. Pretoria, Department of Minerals and Energy, 2006. p. 47. [ Links ]
28. GILFILLAN, A., LESTER, E., CLOKE, M., and Snape, C. The structure and reactivity of density separated coal fractions. Fuel, vol. 78, 1999. pp. 1639-1644. [ Links ]
29. HULSTON, J., CHAFFEE, A.L., BERGINS, C., and STRAUSS K. Comparison of physico-chemical properties of various lignites treated by mechanical thermal expression. Coal Preparation, vol. 25, no. 4, 2005. pp. 269-93. [ Links ]
30. LI, Y., LU, G.Q. and RUDOLPH, V. Compressibility and fractal dimension of fine coal particles in relation to pore structure characterisation using mercury Porosimetry. Particle and Particle Systems Characterization, vol. 16, 1999. pp. 25-31. [ Links ]
31. FAUST, S.D. and ALY, O.M. Chemistry of water treatment. Butterworth, Boston, 1983. 723 pp. [ Links ]
26. PINHEIRO, H.J. A techno-economic and historical review of the South African coal industry in the 19th and 20th centuries and analyses of coal product samples of South African collieries 1998-1999. Bulletin 113. South African Bureau of Standards, Pretoria, 1999. 97 pp. [ Links ]
Paper received Jun. 2010; revised paper received Jun. 2011.
© The Southern African Institute of Mining and Metallurgy, 2011. SA ISSN 0038-223X/3.00 + 0.00.














