Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 n.1 Johannesburg Jan. 2012
JOURNAL PAPER
Kell hydrometallurgical process for extraction of platinum group metals and base metals from flotation concentrates
K.S. LiddellI; M.D. AdamsII
ILifezone SA Ventures Ltd, Switzerland
IIMutis Liber Pty Ltd, Australia
SYNOPSIS
The Kell Process has been developed for the extraction of platinum group metals (PGMs)and base metals from sulphide flotation concentrates. The process has been successfully tested on several different sulphide flotation concentrates, including those from the UG2 chromitite horizon and the Platreef mafic/ultramafic layer. It has been shown to provide high (>95 per cent) and selective extraction efficiencies for the key valuable metals, i.e. Pt, Pd, Rh, Au, Ni, Co, and Cu. The Kell Process consists of several commercially proven unit operations. S, Ni, Co, and Cu are first selectively removed by use of a pressure oxidation step during which the dissolution of PGMs is minimized. The residue from pressure oxidation is subjected to a thermal treatment to ensure efficient PGM recovery by subsequent chlorination. All the core steps are very similar to well-proven conventional unit operations in common use, as are the subsequent metal recovery steps to provide marketable end products. Typical metallurgical responses of flotation concentrates from UG2 and Platreef to the Kell Process are provided, and key outcomes of an energy comparison study with smelting are summarized in this paper. Kell presents a potentially substantial improvement in PGM concentrate processing technology, in terms of economics via much reduced power costs, ease of processing, and various environmental benefits. It allows for the treatment of high-chromium low-grade 'dirty' concentrates, such as secondary concentrates from the platinum industry's 'mill-float-mill-float' (MF2) flotation circuits and concentrates from retreatment of tailings. It allows greater concentrate mass pulls, has higher tolerance to gangue intergrowths in concentrates, and its use can provide substantial increases in overall PGM recovery. Its adoption would be a step change in the platinum industry, and given the commercially proven unit operations embodied in the Kell Process, at much-reduced risk compared with other more experimental technologies. Since the initial process development work, significant improvements and refinements have been introduced as a result of further comprehensive testing and process modelling. Pilot-scale testing and engineering study work is in progress for several selected sites.
Keywords: Kell, platinum, palladium, rhodium, PGM, nickel, copper, UG2, Merensky, Platreef, concentrate, flotation, smelting, matte, hydrometallurgy, leaching, pressure oxidation, roasting, chlorination, refining, metal recovery, energy consumption.
Introduction
As resource companies evaluate and process increasingly complex polymetallic orebodies against a background of higher energy costs and more stringent environmental and carbon emission compliance, so does the metallurgical challenge increase. This is particularly the case for ores containing platinum group metals (PGMs) together with other valuable metals such as gold, nickel, cobalt, and copper. PGMcontaining sulphide concentrates increasingly present challenges to processing by matte smelting, for example when refractory constituents such as chromite are present. The high electrical power demand of PGM smelters and increasing energy costs in South Africa warrant consideration of alternative processing routes that are less energy-intensive and more able to deal with lower quality concentrates.
South Africa is the location of the world's largest resource of PGM1 and from January to October 2011 produced 57 per cent of the total world's mined Pt+Pd+Rh2. South African PGM smelters are under pressure to accept concentrate blends containing greater proportions of concentrates arising from the high-chromite UG2 and the lower-grade Platreef ores, and less from the traditional Merensky Reef, which is historically the backbone concentrate feed to PGM smelters. The impact of higher chromite contents and lower PGM grades on smelter furnace efficiency and operating costs is of prime importance to the ongoing sustainability of the PGM industry.
The mineralogy of PGM ores is complex, with the PGM deporting as fine-grained (often <10 µm) polymetallic phases consisting of PGM sulphides, amphoterics (bismuthides, tellurides, arsenides) and alloys with other metals. The PGM minerals present in the ore have varying associations with base metal sulphide minerals and gangue minerals. The trend towards maximizing recovery from the flotation concentrator requires increased liberation of values from the gangue minerals and this has seen a move to increasingly finer grind sizes3. Fine gangue particles tend to report to the concentrate more readily than coarse gangue particles. Consequently the production of 'smeltable' concentrates becomes more challenging and constrained by the installed capacity and operating parameters of existing smelters. The main example of this is in their ability to deal with the amount of chromite contained in the concentrates, for which each particular furnace has a finite limit.
The current trend of increasing power costs in South Africa may have a large impact on continuing economic viability of individual PGM operations, leading to a significant industry focus on energy efficiency of the PGM processing operations. Rule et al4 and Esterhuizen5 suggest that the tenuous power availability situation in South Africa is not expected to improve for several years due to the long lead times for the installation of new thermal power generation facilities. Electricity is the single biggest input cost for smelting PGM concentrate in an electric furnace, currently exceeding 25 per cent of cash operating costs6. The increasing electricity costs and power restrictions raise the barriers to entry for smaller PGM producers seeking to mine ore or reprocess tailings. Historically, junior PGM producers have sold their flotation concentrates to the major producers, who have utilized their excess smelting and refining capacity as a profitable adjunct to their businesses. While this allows juniors to commence production without having to invest capital in smelting and refining processes, the benefit is offset in the longer term by payments for gross metal value of the concentrate generally being less than 80 per cent of the intrinsic value. Penalties are applied to concentrates not meeting agreed specifications such as PGM grade and chromite content. Most juniors produce predominantly from UG2, and with the smelter-owning majors mining an increasing amount of UG2 their smelters are becoming increasingly chromite-limited. This will over time reduce the toll smelting opportunities for UG2 concentrates. Also, during times of energy restrictions being applied by Eskom to the mining industry the majors will process their own concentrates in preference to those from the juniors. This may cause distortions in their cash flows and higher risk for the junior PGM developers and producers.
There is potentially considerable technical and economic upside to be gained from the application of a robust hydrometallurgical processing route to process PGM concentrates. The ability of such a processing route to handle lowergrade concentrates containing elements deleterious to conventional smelting offers several flowsheet possibilities. These include supplementing the drying/smelting/converting stages for owners of existing smelter facilities and treatment of the entire concentrate stream for producers that have their concentrates toll-treated. Of particular interest is the hydrometallurgical treatment of low-grade concentrates and middling streams produced at some operations, which are currently blended with higher-grade concentrates in an increasingly difficult balancing exercise to produce smeltercompliant feed. Treatment of these streams outside the smelting route would also allow the milling and flotation circuits to be operated 'harder', by grinding finer and targeting a higher mass recovery to concentrate. Operators could thereby increase flotation recoveries in some instances by up to 10 per cent.
The smelting challenge
The mineralogical composition of UG2 concentrate results in an increased energy input requirement to melt it. Using the chemical compositions for Merensky and UG2 concentrates, Van Manen6 has determined energy consumptions to melt the concentrates based on enthalpy calculations. He shows that melting UG2 concentrate requires 667 kWh/t (excluding furnace inefficiencies and heat losses), this being 20 per cent greater than for Merensky concentrate. Pilot smelting of individual concentrates at Mintek in their 200 kW furnace, where system heat losses are expected to be higher than production furnaces, required 896 kWh/t for Merensky concentrate and 1088 kWh/t (21 per cent more) for UG2 concentrate3.
The PGM industry operates a number of electricresistance alternating-current submerged-arc furnaces that are either designed or modified to accommodate high (50 to 80 per cent) proportions of UG2 concentrate. These furnaces have slag tapping temperatures 100ºC to 200ºC higher than those operating on a Merensky-rich feed. This higher slag temperature is due to the higher chromite content in concentrate, which increases the melting point of the slag. Smelting UG2-rich concentrate requires a higher power intensity, further increasing the energy consumption per ton of concentrate. The higher power intensity is necessary to prevent exsolution of chrome oxide spinels from the molten slag as an unwanted phase lying between the slag and matte layers, with its high conductivity impairing the heating of the matte below.
Blending of some Merensky or Platreef concentrates with UG2 is practiced to maintain the Cr2O3 content below a critical level. Strict controls are necessary to ensure that the composition of the furnace feed does not exceed the chromite limit, which causes furnace operations to become unmanageable by affecting the electrical and heat balance requirements necessary to maintain separation of matte and slag within the furnace. For some operations, lower PGM recovery within the UG2 concentrator is accepted in order to keep the chromite level in the concentrate below the smelter plant's limits; other operations have to limit the amount of UG2 that is mined.
UG2-predominant feed is smelted in a number of six-inline electrode rectangular furnaces (19.5 MVA and 68 MVA) having energy consumptions of 800 to 850 kWh/t, and in a 28 MVA 3-electrode circular furnace consuming7 850 kWh/t. The energy consumptions associated with concentrate drying, slag cleaning, gas cleaning, and converting of the furnace matte also have to be added to the overall energy consumed by smelting concentrates; milling of converter matte for the refining stage is also a large consumer of energy. The application of direct-current (DC) arc smelting to UG2 concentrates is not expected to provide energy savings8. Geldenhuys and Jones9 report that four years' operation of a 1.5 MVA DC arc furnace smelting oxide PGM materials arising from smelter reverts in a number of campaigns resulted in energy consumptions, including furnace heat losses, ranging from 834 to 1 218 kWh/t, with an average of 897 kWh/t. The resultant specific energy consumption (the 'melting energy' exclusive of furnace heat losses) of 631 kWh/t was similar to that calculated by Van Manen6 for UG2 concentrate. DC smelting of concentrate with iron-rich alloy collection of PGMs requires roasting of the concentrates to remove sulphur if produced from processing of ore8,9, with the associated significant cost requirement of an acid plant installation.
Kell process
The Kell hydrometallurgical process route presented in this paper was developed specifically for PGM-bearing concentrates and has grant of letters patent by the South African10, USA11, and Canadian12 patent offices.
This process consists of a number of conventional sequential unit operations (Figure 1). Sulphur, Ni, Co, and Cu are first selectively removed by use of a modified pressure oxidation (POX) step. The residue from this step is subjected to a thermal treatment to render PGM phases readily amenable to recovery by chlorination. All the steps are very similar to well-proven conventional unit operations in common use, as are the subsequent metal recovery steps to provide the saleable end-products. Solution streams are typically recycled and reagents are regenerated.
The chromite-related issues encountered in smelting do not pertain to the Kell Process, which is insensitive to the chromite content of the concentrates. Flotation concentrator operations are therefore not constrained by a chromite content limit on the final concentrate and can be operated to maximise PGM recovery.
A comparative energy balance study of the Kell and the matte smelting processes shows that a 50 per cent reduction in energy consumption may be achieved compared with smelting for an 80:20 blend of UG2: Merensky concentrates13. In addition, the electricity consumption is reduced by 82 per cent and the reduction in overall CO2 emissions is 70 per cent. For a treatment rate of 25 t/h concentrate the installed electrical power requirement for Kell is approximately 3 MW compared with approximately 38 MW for matte smelting. The study demonstrated that the energy costs for Kell are R140 per ton of concentrate, compared with R580 per ton for matte smelting and refining.
The Kell process has been successfully tested on several different sulphide concentrates, including those from UG2. It has been shown to provide selective and high extraction efficiencies for the key valuable metals-Pt, Pd, Rh, Au, Ni, Co, Cu. Significant improvements and refinements have also been introduced as a result of further comprehensive batch and pilot-scale test-work and engineering studies undertaken during the past few years on several different concentrate types.
Development of the Kell process
The Kell Process was conceptualized by Liddell to treat UG2 concentrates in 1996, since at that time there was not an established market in South Africa for toll treatment of PGM concentrates. Following flowsheet development and literature review, an extensive test work programme was commissioned to provide proof-of-concept testing on each of the major unit operations. A key fundamental of the process design was to completely separate the sulphate leach for sulphide and base metal removal from the chloride leach for PGM recovery. This separation allows conventional unit operations to be utilized and simplifies process design, engineering, and selection of materials of construction. Testing was carried out under contract by Gipro Nickel Institute JS and All-Russian R&D Institute of Chemical Technology as directed by Liddell. The test work was carried out on two UG2 concentrates, with sulphide contents of 1.7 per cent and 7 per cent and Cr2O3 contents of 2.8 per cent and 1.2 per cent, respectively. Total PGM contents were ~300 g/t and ~1 200 g/t, respectively. Moderate POX temperatures (~150ºC) were used for the sulphate aqueous oxidative leach, without further optimization. Thermolysis temperatures, conditions and residence times were studied in some detail and it is found that the parameters around the intermediate thermal treatment step typically require concentrate-specific optimization to allow for differences in PGM and gangue mineralogy. PGMs were recovered into solution from the roaster calcine in a two-stage chlorination leach and adsorbed onto ion exchange resin that was incinerated to produce a final high-grade PGM concentrate containing 80 to 85 per cent total PGMs plus gold. Recoveries of some 98 per cent were obtained for nickel, 80 per cent for copper, and overall PGM recoveries exceeded 95 per cent. This work is discussed by Tatarnikov et al14.
A process engineering analysis was conducted by Bateman Minerals and Industrial Limited (Bateman) in 1999 on the flowsheet that was derived from the initial test work15. Their scope was to undertake a fatal flaw analysis and advise on improvements and modifications that could be made to optimize the process for specifically South African conditions. Bateman concluded that the process route appeared to have no fatal flaws, and made suggestions pertaining to current design and operating practices of the base metal and PGM refining sections that could be applied to Kell. These recommendations were incorporated into subsequent testing and process design.
More recently the Kell Process was subjected to extensive sequential testing and comparison against some twenty-two alternative flow sheet variations16, including several other hydrometallurgical approaches and variants thereof17,18,19,20,21. This work was carried out using a sample of concentrate prepared from low-grade Platreef ore arising from the northern limb of the Bushveld Complex. The concentrate had a sulphide content of ~9 per cent, Cr content of ~0.1 per cent, Ni+Cu+Co ~3 per cent, Fe ~18 per cent, and total PGM content of ~5 g/t, which represented a more than tenfold PGM upgrade from the ore. The base metals (Ni, Co and Cu) and PGMs were produced as separate solution streams according to the process design. The Kell Process was demonstrated to result in recoveries of over 95 per cent for the PGMs and base metals, which was higher than the alternative flowsheet variations tested, including those embodied by other technologies. The process was hence developed further, with various modifications tested for application to base metals and to lower-grade and 'dirtier' PGM concentrates.
Currently, several PGM concentrate types from a range of operations, ore types and flotation streams are under investigation through programmes of amenability testing, batch-test optimization and pilot-scale testing. A complete simulation model has been compiled by Simulus Pty Ltd13 that can model the mass, chemical, and energy balances using data derived from amenability testing. The Simulus model is used to develop operating and capital costs, energy and reagent consumptions, and equipment specifications. It is envisaged that industrialization of the process will occur through the installation of a demonstration plant processing flotation concentrate at a site facility at significant scale, followed by a definitive feasibility study to generate the investment case. However, a comparatively low scale-up ratio (~500, compared with ~8 000-20 000 industry average22) from pilot scale would in some cases apply.
Kell Process unit components
A general description of the key unit operations inherent to the Kell Process follows.
Pressure oxidation
Pressure oxidation of sulphide concentrates has become firmly established as routine industrial practice in the precious metals and base metals industries as a processing alternative to conventional smelting22,23. Distinct advantages to the POX approach compared with smelting options are many-fewer noxious and greenhouse gases are produced; there is no need for a capital-intensive sulphuric acid plant; energy is often produced in the process (depending on the sulphide mineralogy and content). This unit process has enjoyed commercial operation on a wide variety of feed concentrates, thereby reducing the risk of variability effects and changing ore types.
In the POX autoclave the following reactions are possible for pentlandite, chalcopyrite, and cobaltiferous pyrite - the minerals commonly associated with PGM mineralization:

where M = Ni, Co, Cu. The conversions to elemental sulphur for these reactions are 100 per cent, 50 per cent, and 0 per cent, respectively. Chloride may affect the conversion efficiencies.
The following reactions are possible for pyrrhotite, which is also seen in PGM ores:

The conversions to elemental sulphur for Reactions [4] and [5] are 100 per cent and 12.5 per cent, respectively. Chloride may affect the conversions.
The ferrous sulphate produced is oxidized to ferric sulphate according to the following reaction:
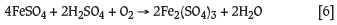
The ferric sulphate may be hydrolyzed to goethite as follows:

Ferric sulphate hydrolyzes to haematite at higher temperatures and lower free acidity via:
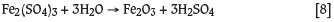
Ferric sulphate hydrolyzes to a basic iron sulphate compound at lower temperatures and higher free acidity via a reversible equilibrium reaction:

Conventional POX for the treatment of sulphide concentrates typically entails operation at 200ºC to 225ºC at ~3 000 kPa for a relatively short residence time of 20 to 45 minutes and results in almost complete sulphide oxidation, with reaction [3] predominating. If sufficient feed sulphide is present, the reaction may be autogenous, providing sufficient heat to sustain itself without resorting to external heating. Moreover, the acid produced in reaction [3] may be sufficient to fulfill the acid demand for the leaching, augmenting the acid in the recycle solution from the base metal recovery section that serves also to polish the mineral surfaces, thereby increasing the leach kinetics even further.
Reducing the operating temperature typically increases the residence time and also results in some partial oxidation of sulphide to elemental sulphur, as depicted by reactions [1] and [2] - this is the case for the Activox® process21, for example, which utilizes an ultrafine grind to around 10 µm followed by a ~110ºC autoclave leach. Also using lower temperature and pressure are the Albion® process24 utilizing ultra fine grinding and chloride addition at ambient temperatures and the LeachoxTM process25. While the potential of ultrafine grind and mild temperature processes may be of generic interest in the context of recovering PGMs, complete sulphide oxidation to sulphate by conventional POX is preferred as a unit operation in the Kell Process to avoid the need to introduce an acid plant to remove sulphur from the roaster off-gases and to achieve high PGM recoveries.
For purposes of comparison it is pertinent to mention Platsol®, which is a high-temperature (>200ºC) pressureoxidation sulphate-based process19,20 with additions of 5 to 20 g/l NaCl into the sulphate media to promote dissolution of some of the PGM minerals. Under those conditions, the base metals (Cu, Ni, Co) and the precious metals (Au, Pt, Pd, etc.) are co-dissolved in a single step. The PGMs are then recovered from solution using sulphide precipitation, activated carbon, or ion exchange. Disadvantages include potential incomplete leach recoveries of PGMs from more refractory mineral phases16, and carry-over losses of PGMs and base metals at the metals separation and recovery stages, due to co-precipitation and adsorption effects. In addition, it is difficult to optimize conditions independently for each value metal species to maximize their dissolution and recovery.
In contrast, conditions in the Kell Process are such that only the base metals are dissolved in the POX stage, and the POX pregnant leach solution from solid-liquid separation can be easily treated by selection of conventional process routes for recovery of metal or intermediate products, such as mixed-sulphide precipitation (MSP)26, mixed hydroxide precipitation (MHP)27, direct solvent extraction (SX)21, crystallization of sulfate salts28 and electrowinning to metal28. The base metal process streams from the Kell Process can be integrated into the existing refining operations of the PGM producers. Typical base metal refinery (BMR) processing29,30 uses the Sherritt Process, comprising atmospheric leach of matte followed by medium (140-150ºC) pressure leach to remove nickel then copper from the PGMrich material. Alternatively, major producers could pass the base metal pregnant leach solution (PLS) from the Kell POX stage directly to their BMR for processing after concentration and/or polishing.
Thermal treatment
The POX residue stream is reduced in mass flow, having removed from the concentrate most of the sulfur, base metals, and some of the iron and gangue species, the amounts depending on the POX and quench conditions. After subsequent solid-liquid separation, the PGM-bearing POX residue can be readily subjected to thermal treatment, in the form of a thermolysis step targeting the chemical decomposition of refractory and complex PGM-bearing minerals into readily leachable forms. Modern technologies such as fluidbed and circulating fluid-bed roasters as well as rotary kilns are extensively utilized at commercial scale29,30. While the off-gases in this application are relatively benign because most of the sulfur is removed in the POX step, osmium and iridium may potentially be recovered. Moreover, removal or stabilization of selenium, tellurium and arsenic may occur under appropriate conditions. This represents a potential environmental benefit as well as a reduction in common deleterious elements present in conventional PGM refining of smelter matte. The use of heat and energy recovery methods to integrate the heat balance of the thermal treatment with POX, PGM leaching and reagent regeneration stages further reduces the overall energy consumption and CO2 footprint of the Kell Process13.
The PGM mineral phases in concentrates are often complex moieties such as Pd-Bi-As phases (amphoterics), refractory PtS minerals and precious metal tellurides. Temperatures required for converting PGM minerals into readily leachable forms are typically 800 to 1000ºC for short residence times and present no major practical design or operational difficulties. The atmosphere can be selected and controlled for reducing or oxidizing conditions, dependent on the concentrate mineralogy and available fuel source; this effect has been subject to study16.
Chlorination leaching
Leaching of PGMs requires the high oxidation-reduction potentials (ORPs) and complexing ligand provided by chlorination leaching; for this reason, and to exploit the differences in the chemistry of their anionic chloro-complexes in separation of the PGMs, chlorination leaching has become a standard unit process in PGM refineries29,31. Use of chlorination leaching by the Kell Process again represents the application of industry-standard and well-established technology having many years of commercial application. For junior producers who do not have their own smelter or refinery, processing concentrates at their mine site using the Kell Process and closing the circuit with ion exchange resin or other means for the recovery of a high-grade PGM concentrate product and reagent recovery provides an efficient circuit for ultimate production of an internationally saleable high-grade product. Alternatively, for major producers, the chloride solutions can pass directly to an existing PGM refinery circuit after concentration and polishing.
The three main process steps of POX, thermal treatment, and chloride leaching can be decoupled from each other to suit individual site conditions, providing maximum flexibility for concentrate producers to utilize existing base metals and PGM refineries if they have them in their flowsheet.
Conclusion
The Kell Process for recovery of base metals and PGM into separate solution streams for downstream conventional refining has seen significant development work and comparative testing. It presents a potentially substantial advance in PGM concentrate processing technology, in terms of economics via much reduced power costs, ease of processing, and various environmental benefits. Given the current trend of increasing environmental accountability and power costs, these benefits are of utmost importance.
The Kell Process can treat high-chromium, low-grade 'dirty' concentrates, such as secondary concentrates from MF2 flotation circuits, middlings streams, and concentrates from retreatment of tailings impoundments. It potentially allows greater concentrate mass recoveries, higher tolerance to gangue intergrowths, and substantial increases in overall PGM recovery.
Kell has the potential to be a technological step change in the platinum industry, and given the commercially proven unit operations embodied in the process, at much reduced risk compared with other more experimental technologies.
References
1. STILWELL, L.C. and MINNITT, R.C.A. Is platinum paying its rent? International Platinum Conference 'Platinum Surges Ahead'. The Southern African Institute of Mining and Metallurgy, Johannesburg 2006. pp. 285-293. [ Links ]
2. BUTLER, J. Platinum 2011 Interim Review, Johnson Matthey plc, November 2011. [ Links ]
3. LIDDELL, K.S., MCRAE, L.B, and DUNNE, R.C. Process routes for the beneficiation of noble metals from Merensky and UG-2 ores. Extraction Metallurgy '85, London, UK. Institution of Mining and Metallurgy, London, 1985. pp. 789-816. [ Links ]
4. RULE, C.M. Energy considerations in the current PGM processing flowsheet utilizing new technologies. Journal of the Southern African Institute of Mining and Metallurgy, vol. 109, no. 1, 2009. pp. 39-46. [ Links ]
5. ESTERHUIZEN, L. Power versus PGMs - Eskom wins. Royal Bank of Canada Europe Ltd. Journal of the Southern African Institute of Mining and Metallurgy, 11 April 2008. 56 pp. [ Links ]
6. VAN MANEN, P.K. Furnace energy efficiency at Polokwane smelter. Journal of the Southern African Institute of Mining and Metallurgy, vol. 109, no. 1, 2009. pp. 47-52. [ Links ]
7. JONES, R.T. An overview of southern African PGM smelting. Nickel and Cobalt 2005: Challenges in Extraction and Production, 44th Annual Conference of Metallurgists, Calgary, Alberta, Canada, 21-24 August 2005. CIM, Montreal, 2005. pp. 147-178. [ Links ]
8. ESTERHUIZEN, L. PGMs & South African golds trip notes - who needs what when. Royal Bank of Canada Europe Ltd. 19 February 2010. 40 pp. [ Links ]
9. GELDENHUYS, I.J. and JONES, R.T. Four years of DC arc smelting of PGMcontaining oxide feed materials at Mintek. Mintek, Randburg, South Africa, 2009. 32 pp. [ Links ]
10. LIDDELL, K.S. Hydrometallurgical treatment process for extraction of platinum group metals obviating the matte smelting process. S. Afr. Pat. 2000/6600: Appl. 19 May 1998: Acc. July 25 2001. [ Links ]
11. LIDDELL, K.S. Hydrometallurgical treatment process for extraction of platinum group metals obviating the matte smelting process, US Pat. 6,579,504: Appl.19 May 1999: Acc. 17 June, 2003. [ Links ]
12. LIDDELL, K.S. Hydrometallurgical treatment process for extraction of platinum group metals obviating the matte smelting process, Canada Pat. 2,332,520: Appl. 19 May 1999: Acc. 27 January, 2009. [ Links ]
13. LIDDELL, K.S., NEWTON, T., ADAMS, M.D. and MULLER, B. Energy consumptions for Kell hydrometallurgical refining versus conventional pyrometallurgical smelting and refining of PGM concentrates. The 4th International Platinum Conference, Platinum in transition 'Boom or Bust'. Sun City, South Africa. The Southern African Institute of Mining and Metallurgy, October 2010. pp. 181-186. Journal of the Southern African Institute of Mining and Metallurgy, vol. 111, February 2011. pp. 127-132. [ Links ]
14. TATARNIKOV, A.V., SOKOLSKAYA, I., SHNEERSON, YA. M., YU. LAPIN, A., and GONCHAROV, P.M. Treatment of platinum flotation products. Platinum Metals Review, vol. 48, no. 3, 2004. pp. 125-132. [ Links ]
15. BATEMAN MINERALS AND INDUSTRIAL LTD. Confidential Report. Boksburg, South Africa. May 1999. [ Links ]
16. ADAMS, M.D., LIDDELL, K.S., and HOLOHAN, T.N. Hydrometallurgical processing of Platreef flotation concentrate. Proceedings of Precious Metals 10, Falmouth, UK 15-16 June 2010. Minerals Engineering, 2011, vol. 24, pp. 545-550. [ Links ]
17. MOYES, J. and HOULLIS, F. The development of the Intec Nickel process to treat a low-grade Ni/Co/Cu/PGM concentrate. ALTA 2003 Ni/Co-9 Conference Proceedings. ALTA Metallurgical Services, Melbourne. 2003. [ Links ]
18. GREEN, B.R., SMIT, D.M.C., MAUMELA, H., and COETZER, G. Leaching and recovery of platinum group metals from UG-2 concentrates. Journal of the Southern African Institute of Mining and Metallurgy, vol. 104, no. 6, 2004. pp. 323-332. [ Links ]
19. DREISINGER, D. Hydrometallurgical process development for complex ores and concentrates. Journal of the Southern African Institute of Mining and Metallurgy, vol. 109, no. 5, 2009. pp. 253-271. [ Links ]
20. FLEMING, C.A., FERRON, C.J., DREISINGER, D.B., and O'KANE, P.T. A process for the simultaneous leaching and recovery of gold, platinum group metals and base metals from ores and concentrate. EPD Proceedings, TMS Annual Meeting, Nashville, 2000. The Minerals, Metal and Materials Society, Warrendale, Pennsylvania. pp. 419-431. [ Links ]
21. ADAMS, M.D. and JOHNSON, G.J. Advances in nickel sulphide process development. ALTA Ni/Co-7 Conference Proceedings. 2001. ALTA Metallurgical Services, Melbourne. 13 pp. [ Links ]
22. ADAMS, M.D., VAN DER MEULEN, D.R., LUNT, D.J. and ANDERSON, P. Selection of piloting parameters in pressure hydrometallurgy. Metallurgical Plant Design and Operating Strategies. 2004. Australian Institute of Mining and Metallurgy, Melbourne. pp. 543-555. [ Links ]
23. THOMAS, K.G. Pressure oxidation overview. Advances in Gold Ore Processing. Adams, M.D. (ed.) Elsevier, Amsterdam, 2005. pp. 346-370. [ Links ]
24. HOURN, M., MACDONALD, C.A., ROHNER, P., and WOODALL, P. Albion process and leaching high arsenic materials at Mt Isa. ALTA Copper-10 Conference Proceedings. ALTA Metallurgical Services, Melbourne, 2006. 19 pp. [ Links ]
25. FLATMAN, S., BATTERSBY, R.M., IMHOF, R., BATTERSBY, M., and IBRAYEV S. The Leachox™ refractory gold process - the testing, design installation and commissioning of a large scale plant at the Vasgold Gold Mine, Kazakhstan. Precious Metals 10. Minerals Engineering International. Falmouth, UK, 2010. [ Links ]
26. ADAMS, M.D., et al. A complete approach to complex flowsheet development-Niquel Do Vermelho (CVRD) case study. International Laterite Nickel Conference. 2004. The Minerals, Metal and Materials Society, Warrendale, Pennsylvania. pp. 161-169. [ Links ]
27. ADAMS, M.D., et al. Piloting of the beneficiation and EPAL® circuits for Ravensthorpe Nickel Operations. International Laterite Nickel Conference. 2004. The Minerals, Metal and Materials Society. Warrendale, Pennsylvania. pp. 193-202. [ Links ]
28. LONMIN PLC. Process Division Presentation. June 2010. [ Links ]
29. CRAMER, L.A. The extractive metallurgy of South Africa's platinum ores. Jom, vol. 53, no. 10, 2001. pp. 14-18. [ Links ]
30. RADEMAN, J.A.M., LORENZEN, L., and VAN DEVENTER, J.S.J. The leaching characteristics of Ni-Cu matte in the acid-oxygen pressure leach process at Impala Platinum. Hydrometallurgy, vol. 52, 1999. pp. 231-252. [ Links ]
31. THOMAS, K.G. and COLE, A.P. Roasting developments-especially oxygenated roasting. Advances in Gold Ore Processing. Adams, M.D. (ed.). Elsevier, Amsterdam, 2005. pp. 403-432. [ Links ]
32. HAMMERSCHMIDT, J., GÜNTNER, J. and KERSTIENS, B. Roasting of gold ore in the circulating fluidized-bed technology. Advances in Gold Ore Processing. Adams, M.D. (ed.). Elsevier, Amsterdam, 2005. pp. 433-455. [ Links ]
31. NEWELL, A.J. The processing of PGM-part 2. Pincock Allen & Holt. May 2008. 4 pp. [ Links ]
Paper received Mar. 2010; revised paper received Dec. 2011.
© The Southern African Institute of Mining and Metallurgy, 2011. SA ISSN 0038-223X/3.00 + 0.00.














