Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.111 no.11 Johannesburg nov. 2011
JOURNAL PAPER
Fluidized bed roasting of micropelletized zinc concentrate: part I- pellet strength and roasting kinetics
S. Heukelman; D. Groot
Department of Materials Science and Metallurgical Engineering, University of Pretoria, Pretoria, South Africa
SYNOPSIS
Zincor, a zinc refinery in South Africa, uses oxygen enrichment of up to 26% O2 in its fluidizing air to increase concentrate-throughput in its fluidized bed roasters. The aim of the study is to determine whether O2 enrichment can be reduced by introducing micropelletized concentrate into the feed blend, while maintaining concentrate throughput rates and calcine quality. A laboratory scale fluidized roaster was used to determine the strength and oxidation kinetics of suitable micro-pellets. The strength of the pellets was determined by the extent of attrition during roasting. It was found that micro-pelletization decreases the fines in the feed to the roasters; the -500 µm fraction decreased from 87% to 10%. The micro-pelletized particles do break down during roasting, which increases the -500 µm fraction to 31%. The general morphologies of the roasted particles are similar to those found by previous workers. Micro-pelletized concentrate particles require more time than nonpelletized concentrate particles to oxidize in the roasting step, although this is less than the mean residence time of the Zincor roasters. Roasting micro-pellets in O2-enriched fluidizing air increased the reaction rate.
Keywords: O2 enrichment, fluidizing air, zinc concentrates, morphologies, ZnO and ZnFe2O4, micro-pelletization, particle breakdown and attrition, residence time, conversion and reaction rate.
Introduction
Oxidation of zinc concentrate in fluidized bed roasting
Zincor is a zinc refinery to the east of Johannesburg in South Africa. The process used is described in Part II of this paper1. Historically, since fluidized beds have been used, air has been the oxidizing medium to convert ZnS to ZnO. In an attempt to increase roaster throughput Air Products and Chemicals, Inc. (APCI) were the first to introduce O2 enrichment into the fluidized air in April 19832. Zincor followed suit in 1996 and use up to 26% O2-enrichment (21% O2 in air and 5% O2 from a 99.5% O2 source) to increase throughput capabilities3. O2 enrichment increases the rate of the oxidation reaction4,
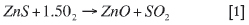
and therefore feed rates to the roasters can be increased. The enrichment allows the same total flow rate of fluidizing gas, and thus the loss of fines through entrainment is not increased.
O2 enrichment allows roaster throughput to be increased, while the feed particle-size distribution can be maintained. If, however, a coarser feed particle size could be used, the flow rate of fluidizing air could be increased, also allowing an increased roaster throughput. This is feasible, as the fluidizing air supplies O2 in excess of the stoichiometric requirements. One way of increasing the feed particle sizes is through full or partial pelletization of the feed. However, as will be shown later, larger particles require more time to convert to ZnO, and thus the maximum size of such pellets would have to be controlled. The resistance to attrition and breakdown of the pellets will also have to be maximized.
Transformation processes during roasting
The phase transformation processes occurring when zinc concentrate is oxidized have been well researched. Graydon and Kirk5 give a good description of the phase transformation process when zinc concentrate is roasted. FeS2 phases in solid solution with ZnS oxidize first. The first stage is complete with a shell of Fe3O4 and oxidized ZnO that condenses from the roaster atmosphere onto the outer rim of the particle.
The second stage involves a rise in the O2 partial pressure at the reacting interface and the onset of oxidation of mainly ZnS to ZnO, which forms in solid solution with the remaining oxidized iron as Fe2+. Concurrently, some iron will diffuse from the Fe3O4 rim into the newly formed ZnO. Upon completion of the ZnS oxidation, the O2 partial pressure in the particle rises to the roaster atmosphere pressure, and the remaining Fe2+ in solid solution is oxidized to Fe3+. Simultaneously, the Fe3O4 rim with condensed ZnO is transformed into the refractory zinc ferrite, ZnFe2O4.
In the last stage of the transformation process, the Fe3+ that was formed reacts with ZnO to produce separate ZnFe2O4 and pure ZnO phases.
Chen and Dutrizac6 studied the mineralogical changes that occur when zinc concentrate is roasted. The calcine consisted of unreacted ZnS and uniform, porous, banded masses of (Zn,Fe)O or ZnO and ZnFe2O4. The surface of the particles contains a fine-grained uniform and porous mass of ZnO + ZnFe2O4. A dense (Zn,Fe)O phase appears between the sphalerite and ZnO + ZnFe2O4 layer. Also present are small pores attributed to the evolution of SO2 gas during roasting or differences in density of the sphalerite and the reaction products that form6.
Micro-pellet roasting in this study is expected to follow the same transformation processes. The micro-pellets are larger than the non-pelletized concentrate particles, and incomplete roasting with a remaining ZnS core could result. Should this occur, it would indicate that the particle residence time inside the roaster was insufficient1, if all other control variables (i.e. bed temperature, fluidizing air rate) are kept constant.
Variables affecting micro-pellet roasting
ZnS pelletization
Zinc concentrates were pelletized for a horizontal-type fluidized bed roaster as early as 19547. Subsequently, numerous methods to pelletize zinc concentrates were successfully implemented7-13. The underlying purpose has always been to reduce fines, or to increase bed stability and to reduce entrainment.
Denoiseux et al.7 produced pellets using a granulating drum and controlled the size between 0.5 mm and 4 mm. The sulfur present as either a sulfide (denoted S/S) or a sulfate (denoted S/SO42-) in the roasted material is used to indicate how successfully ZnS was converted to ZnO. In the entrained particles, S/S and S/SO42- were 0.25% and 0.35% higher, respectively, than in particles in the bed overflow. The longer residence time of pellets in the roaster bed was given as reason for improvement in quality of calcine production7. Sanchuan et al.13 produced cylindrical pellets of 8 mm to 10 mm in diameter and 10.26 mm in length. Drying at 105ºC was the main factor contributing to the strength of the pellets. The strength was also attributed to crystallization of ZnSO4 within the pores of the pellets. It was determined that using 3% ZnSO4 as a binder is the optimum amount to increase the pellet strength. Sanchuan et al.13 found high levels of entrainment and incomplete roasting of the sulphides resulted when roasting non-pelletized concentrates with a particular size of 90% passing 75 μm.
Brown and Goosen9 produced pellets with an average particle size of 1500 μm. Entrainment was reduced, so that the bed overflow increased from between 5% and 15% up to between 25% and 35%. Brown8 reported that excessive entrainment could result in inefficient roasting. Feeding pellets to the roaster resulted in less than 0.5% sulfur in the bed overflow and thus better sulfur elimination. Carrey and Hall10 produced agglomerates under high pressure that decrepitated once introduced into the roaster. It was found that by adding approximately 6% ZnSO4 and as little as 0.5% of -10 μm particles, the strength of the agglomerates increased. The strength of agglomerates increased and then decreased as the moisture content was varied from 14.6% to zero. The strength of the agglomerates increased rapidly with increasing temperature of exposure between 200ºC and 1000ºC.
Zincor uses a simplified method of ZnS concentrate pelletization by increasing the contained moisture content of the feed up to 10%. Previous test work undertaken to improve roasting operations established this operating practice14. The 18 m2 roaster design feed rate is 117 t/day, and had been increased to 126 t/day. The main factor that enabled this increase was controlling the feed concentrate moisture content between 8% and 9%, with only air as the fluidizing medium.
Zincor controls the content of > 1 mm particles to less than 20% to maintain optimum fluidization conditions, and it will therefore be beneficial to minimize larger-sized particles when pelletization is introduced. The ideal pellet size for this study is +0.5 to -1 mm. Figure 1 shows the design particlesize distribution for the bed15.
This limit was determined based on a high rate of bed failures during the mid-1980s to 1990s. It is well above the design distribution of +1 mm particles, accounting for approximately 10% of the feed. This difference shows the robustness of the fluidizing bed.
Different sized pellets react at different rates when exposed to an oxidizing atmosphere14,16,17. Sanchuan et al.13 performed roasting tests at varying temperatures on 1.22 mm and 1.73 mm pellets to determine the time required for complete conversion. As expected the time required for conversion of a 1.73 mm particle exceeded that for the smaller 1.22 mm particle. Natesan and Philbrook18 found that the smaller the pellet the higher the conversion for any given time. Different sized pellets were exposed to 960ºC and 27% O2. The pellet sizes varied from 4.1 mm to 16 mm. It took approximately 25 minutes to convert a 4.1 mm pellet. The time required for oxidation increases substantially with increasing pellet size: at 70 minutes the 16 mm pellet had not reached 60% conversion. Natesan and Philbrook16 also studied the roasting kinetics of various particle sizes of nonpelletized concentrate in a 4-inch laboratory scale fluidized roaster. Roasting was performed in a 1-inch bed depth on particles of 0.18 mm, 0.32 mm, and 1 mm in a 21% O2 atmosphere. Consistent with their earlier study, it was found that the smaller particles are converted at a faster rate.
Oxygen concentration
Cannon and Denbigh's17 study using ZnS crystals showed that an increase in the O2 concentration resulted in an increase in the rate of the oxidation reaction. Sanchuan et al.13 also showed that the reaction rate of ZnS oxidation is proportional to the O2 concentration at 750ºC and 950ºC. Natesan and Philbrook16,18 studied the effect of different O2 levels in the fluidizing air on the reaction rates of nonpelletized and pelletized concentrate. In both instances, it was shown that increased levels of O2 in the fluidizing air increases the rate of the ZnS oxidation reaction.
Current production rates at Zincor can be maintained by enhancing the reaction rates through O2 enrichment. This is due to the short average residence times in industrial roasters1. Residence times may be improved by micro-pelletization, thus possibly reducing the need for O2 enrichment.
Experimental procedure
Micro-pellet production
The first step in the study was to determine the optimum conditions to produce micro-pellets that resist attrition during roasting. Three procedures were tested for the production of suitable micro-pellets:
Increasing the final moisture content
Use of a binder consisting of different ratios of impure ZnSO4 solution and water. Impure ZnSO4 is a product of the neutral leaching step of the roasted calcine. The ZnSO4 concentration was varied between zero and 220 g/l
Use of different pelletizer speed settings.
Micro-pellets were produced with the Loedige KM 300 ploughshare mixer. This pelletizer was chosen based on previous work performed at Zincor. The unit has a capacity of 8 t/h wet feed. It was installed in parallel to the existing concentrate feed system. The main shaft of the pelletizer, which conveys the concentrate from the inlet to the outlet, has two possible speed settings of 70 or 140 r/min. In addition, two chopper motors with a shaft and blades also have a combined two-speed setting of low and high speeds. Samples were taken of the non-pelletized concentrate and of the produced micro-pellets. The samples were stored in sealed bags. The samples were used to determine the feed and product moisture content. The samples were weighed before being dried in a muffle furnace at 120ºC for 24 hour. The dried sample weight was then determined.
Micro-pellet strength tests
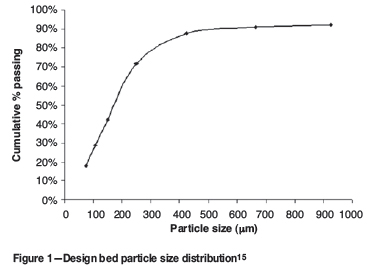
Using compressive strength tests or fall impact tests as used by previous investigators10,13 to test the strength of the micro-pellets would not be practical due to the micro-pellets' size, which might influence the accuracy of the results. Although the bulk of the unpelletized material was smaller than 75 μm, the only requirement was to know the fraction of fines below 500 μm (this is the largest particle that is entrained) and the fraction of coarse material above 1000 μm (this is the largest micro-pellet to be produced to ensure sufficient residence time for conversion)1. To determine if the strength of the micro-pellets would be sufficient to prevent excessive break-up and attrition the micro-pellets were roasted for 1 hour in an air atmosphere in a laboratory scale fluidized roaster controlled at 950ºC. Size distributions were determined for particles before and after roasting. The difference in pelletized and unpelletized particle size distributions was used to determine the strength of the pelletized concentrate. Particle size analysis was performed with sieve sizes 75, 212, 300, 500, 850, 1000, 1400, 2360, 3150, and 4000 μm. The laboratory scale roaster set-up is shown in Figure 2.
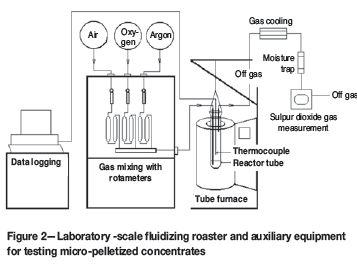
A 20 mm porous-bottom silica glass reactor tube in a thermocouple-controlled vertical-tube furnace was used in all the laboratory tests, as shown in Figure 3.
The reactor tube was placed inside an external silica holding tube with an entry point for the fluidizing gas. The holding tube and the reactor formed a seal at the necks of the two units. This configuration formed a sleeve between the holding tube and the reactor that allows the fluidizing gas to enter the porous bottom of the reactor. The required flow rate and ratio for the preferred fluidizing gas mixture were controlled with rotameters. Only air, O2 or Ar were used during the tests.
The gas exiting the reactor was cooled by passing it through two glass cooling towers on the tube side. Cool pressurized air was passed through the cooling tower on the shell side. The cooled gas then passed through a resin water trap to ensure that the gas was dehydrated and so prevent sulphuric acid formation before entering the SO2 gas analyser. Sulphuric acid will form if trace levels of SO3 and moisture are present in the gas. The off-gas was then released to the atmosphere.
The Ultramat 6F infrared gas analyser, produced by Siemens19, was used to measure the percentage SO2 (v/v) concentration. The analyser was calibrated by first performing a zero calibration with the oxidizing atmosphere for the specific test, and then with a 5% chemical grade SO2 calibration gas. The roaster was set to the required temperature for the specific test and left to stabilize. Prior to each roasting test, the reactor was flushed with Ar for 1 minute to ensure an inert atmosphere. Data logging was started before Ar flushing. The micro-pellets were then placed inside the reactor and the required fluidizing atmosphere was set for the specific test. All tests were performed at a total gas flow rate of 7.6 L/min. This flow rate represents flow conditions present inside a Zincor industrial sized roaster and is equivalent to 500 Nm3/h/m2. Each test was run until no more SO2 was measured. The sample was then removed from the reactor and placed in a sealed polytop container for analysis.
The effects of micro-pellet size and O2 enrichment
Tests were performed to determine the time required to roast different sizes of micro-pellets and to gain a better understanding of how micro-pellets would react inside a roaster. The size of the micro-pellets (1000 μm to 4750 μm) and O2 enrichment (air and 23%, 24.5%, and 26% O2) were varied. The micro-pellets used in the tests were produced with the pelletizer at the settings for optimal strength micropellets. All the tests were conducted at 950ºC using 0.33 g of sample, and each test was done in triplicate. The extent of conversion of the roasted particles was determined by calculation from the original known sulfur content, the measured %SO2 (v/v) and the gas flow rate.
The roasted particles were studied using scanning electron microscopy (SEM) with energy-dispersive X-ray spectroscopy (EDX) analysis to acquire semi-quantitative information about the samples. A JOEL JSM 6300 instrument was used. The samples were set in an epoxy resin and were polished through to the sample for analysis.
Results and discussion
Micro-pellet strength measurement
The results for (1) water only as a binder, (2) pelletizer settings, and (3) ZnSO4 as a binder are discussed in the following section.
Extra test work to confirm repeatability was not possible due to the pelletizer being available to Zincor for only a limited time. However, comparing the trends for the different tests does provide sufficient confidence in the results. The samples used for the test work were taken from a continuous process, which should mitigate some of the variability inherent in batch processes.
Water as a binder
The first phase of the tests was to determine the effect of increasing the moisture content of the micro-pellets. The results are shown in Figure 4. The data points represent single experimental runs.
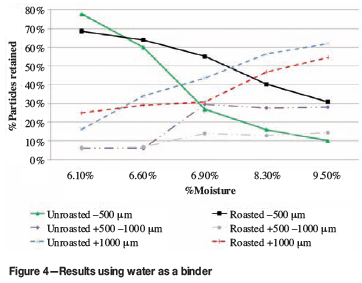
The content of -500 μm particles was reduced from 87% in the unpelletized feed to 31% through micro-pelletization with 9.5% moisture. The results for unroasted particles show that increasing amounts of binder decrease the percentage of fines (-500 μm), with concomitant increases in the coarser fractions. This confirms that moisture plays an important role in forming bigger particles, consistent with findings by previous investigators, as discussed above. Most of the agglomerated particles fall in the size fraction +1000 μm. The trends seen in particle size as a function of binder content for unroasted particles seem largely unchanged by the roasting procedure. There is evidence of attrition, but there are still net gains obtained by increasing the amount of binder. Beyond about 7% moisture, an increasing amount of binder does not seem to result in particles that are more resistant to attrition, as the trends for unroasted and roasted particles are more or less parallel.
Ideally, with an optimized micro-pelletization process, the particles would all be in the +500 -1000 μm size range when leaving the roaster1. Roasting of the +500 -1000 μm particles resulted in attrition, deceasing the content of this size fraction from 28% to 14% at all the moisture levels tested, except at 6.1% and 6.6% moisture.
Producing roasted micro-pellets in this desired size range is as important as not producing excessive amounts of +1000 μm roasted pellets. Attritioning of the +1000 μm micro-pellets during roasting is desirable therefore and beneficial to the overall efficiency of the process by lowering the risk of producing low quality calcine.
At 6.1% moisture, agglomeration of the particles occurred during roasting, which reduced the -500 μm particle content from 78% to 69%.
The particles were roasted for only 1 hour and it is reasonable to assume that further particle attrition will occur during longer periods of roasting. Particles in the bed have a calculated1 average residence time of 4 hours, and longer roasting time might lower the fraction of +1000 μm particles even further. This will be important, recalling that Zincor controls the +1000 μm particles to around 20% to prevent defluidization of the bed.
Pelletizer settings
Subsequently, mechanical settings on the pelletizer were varied for micro-pellets containing levels of moisture above 9%. The results in Table I compare unroasted with roasted micro-pellets.
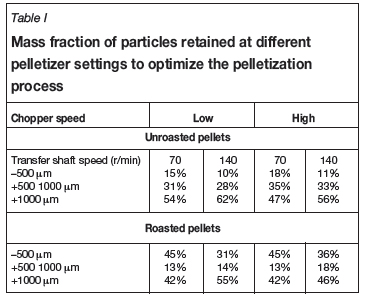
It was found that an appreciable decrease in the amount of fines (-500 μm) resulted from the combination of a high transfer shaft speed and a low chopper speed. The product micro-pellets were mainly in the +1000 μm size fraction. These trends hold for the unroasted pellets and the pellets after the attrition caused by roasting. Most of the loss due to attrition was from the +500-1000 μm size fraction. The A high shaft speed and a low chopper speed resulted in the least degradation by attritioning. Based on these results, a high shaft speed and low chopper speed setting were used to produce the micro-pellets required for the test work.
Impure ZnSO4 electrolyte as a binder
Clear trends in the extent of agglomeration, or the extent of attrition through roasting, were not found. Therefore ZnSO4 solution was not used as a binder to produce micro-pellets.
The particle size distribution for micro-pellets produced at optimal conditions compared with non-pelletized feed is shown in Figure 5.
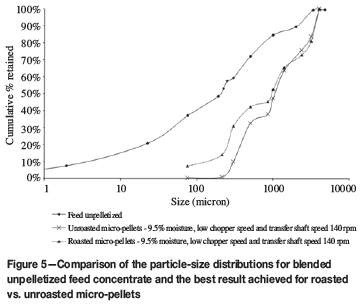
The optimal conditions for micro-pellet production with the pelletizer are moisture levels of 9.5% to 10.5%, a high transfer shaft speed of 140 r/min, and a low chopper speed.
Laboratory-scale micro-pellet roasting
Micro-pellet size
The results for roasting variously sized micro-pellets in air are shown in Figure 6.
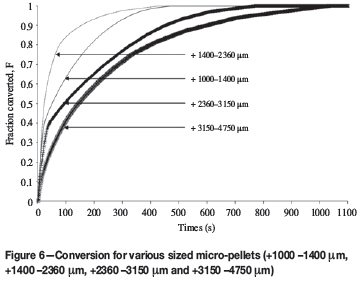
Unexpectedly, the +1400 -2360 μm micro-pellets reached full conversion before the +1000 -1400 μm micro-pellets. This can be explained by particle attrition and degradation of the micro-pellets. These +1400 -2360 μm pellets, for an inexplicable reason, were found to be more prone to degradation and attrition.
The +3150 -4750 μm sized micro-pellets required approximately 17 minutes for full conversion. The result compares reasonably with the Natesan and Philbrook16 study; a single 4.1 mm particle required approximately 25 minute to be converted. Table II shows the measured conversion times and calculated residence times for various particle sizes. The particle sizes used for the residence time calculations differ slightly from the sizes used in the test work, as shown.
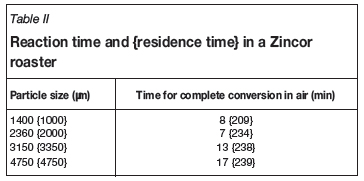
The reaction times shown in these tests are far less than the calculated1 residence times inside a Zincor roaster, or the measured retention times as found by Spira20. Therefore, using micro-pellets should not reduce the specific calcine production rates1 achieved with O2 enrichment.
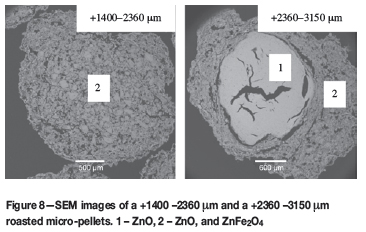
SEM images of an unroasted micro-pellet and roasted micro-pellets are shown in Figures 7 and 8.
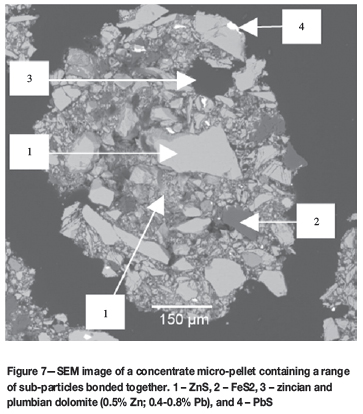
The SEM image in Figure 7 shows a typical concentrate micro-pellet that consists of various small mineral particles randomly bonded together. Zn(Fe)S particles are shown as the light to darker grey particles that constitute the bulk of the particles.
Figure 8 shows that the micro-pellets were completely oxidized. The +1400 -2360 μm micro-pellet shows a typical image of a roasted particle consisting of the more porous ZnO + ZnFe2O4, which passed through the full transformation process. Pores that may be associated with the evolution of SO2 during roasting are visible. The +2360 -3150 μm micropellet is distinctly different, with dense ZnO in the middle surrounded by a ZnO + ZnFe2O4 outer rim. This is a typical roasted particle and consistent with the depiction of Chen and Dutrizac6 of their partially roasted zinc concentrate - phase 2 (Zn,Fe)O and phase 3 ZnO + ZnFe2O4. In general, the morphologies of the roasted particles are similar to those found by Graydon and Kirk5 and by Chen and Dutrizac6.
Oxygen enrichment
The results for the roasting of +1000 -1400 μm pellets in O2enriched fluidizing air are shown in Figure 9.
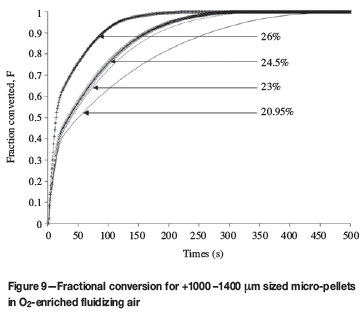
The results show the influence that O2 enrichment has on the reaction rate. It took approximately 8 minutes to roast the micro-pellet to completion in air, compared with approximately 3.5 minutes in 26% O2. The influence that O2 enrichment has on fluidized bed roasting is well known and is used at Zincor.
SEM images of the +1000 -1400 μm micro-pellets roasted in O2-enriched air are shown in Figure 10.

Both particles are from the same test. The particle in Figure 10A once again exhibits a dense ZnO core surrounded by a porous ZnO + ZnFe2O4 mass. The particle in figure 10B consists of only the porous ZnO + ZnFe2O4 mass. The general morphologies of the particles roasted in O2-enriched air are similar to those of particles roasted in air only, as discussed in the previous section.
Discussion and conclusions
Production of micro-pellets and strength measurement
The use of micro-pellets produced with the pelletizer was effective in reducing entrainable particles from 87% to 31%. Water was an effective binder at moisture levels of 9.5%. The study was inconclusive regarding ZnSO4 solution as a binder to increase micro-pellet strength. The strength of +1000 μm pellets was superior to +500 -1000 μm pellets.
The production technique was not efficient in producing mostly the desired +500 -1000 μm size range, and +1000 -4750 μm micro-pellets were preferentially produced. The different mechanical settings on the pelletizer had some influence on micro-pellet production. Using a high shaft speed and low chopper speed setting gave the optimized production conditions for this technique.
Using micro-pellets between +500 -1000 μm is favoured for the Zincor roasting process. Attritioning and particle degradation of the +1000 -4750 μm micro-pellets occurs during roasting, and production of the micro-pellets in this size range can thus be accommodated to a limited extent1.
Laboratory-scale micro-pellet roasting
Laboratory-scale roasting in this study showed that the +3150 -4750 μm sized micro-pellets required approximately 17 minutes to convert fully. The calculated average residence time for particles of this size in the Zincor roasters is 4 hour1. Likewise, 500 μm (2.5 hours) and 1000 μm (3.5 hours) particle residence times far exceed the required reaction times. This indicated that the introduction of micro-pelletized feed would be feasible.
Roasting micro-pellets in O2-enriched fluidizing air increased the reaction rate. In air the +1000 -1400 μm pellets required 8 minutes to convert, while in 26% O2-enriched air they required only 3.5 minutes.
The general morphologies of the roasted particles are similar to those found by Graydon and Kirk5 and Chen and Dutrizac6. Some roasted micro-pelletized samples contained a dense ZnO kernel and a porous ZnO + ZnFe2O4 outer rim mass.
Acknowledgements
I (SH) would like to express my sincere gratitude to Exxaro Resources Limited for allowing me to undertake this study. The paper is published with the permission of Exxaro Resources Limited.
References
1. HEUKELMAN, S. and GROOT, D. Fluidized bed roasting of micro-pelletized zinc concentrate. Part II. Particle entrainment and residence time. Journal of The Southern African Institute of Mining and Metallurg, vol. 111, no. 11, November 2011. pp. 767-772. [ Links ]
2. SAHA, D., BECKER, J.S., and GLUNS, L. Oxygen-enhanced fluid bed roasting. Productivity and Technology in the Metallurgical Industries. Koch, M., and Taylor, J.C. (eds.). The Minerals, Metals and Materials Society, 1989. ISBN 9780873391009. pp 257-280. [ Links ]
3. MACLAGEN, C., CLOETE, M., MEYER, E.H.O., and NEWALL, A. Oxygen enrichment of fluo-solids roasting at Zincor. Proceedings of Lead-Zinc 2000. Dutrizac, J.E., Gonzales, J.A., Henke, D.M., James, S.E., and Siegemund, A.H-J. (eds). Warrendale, PA, The Minerals, Metals and Materials Society, 2000. pp. 417-426. [ Links ]
4. KUNII, D. and LEVENSPIEL, O. Fluidization Engineering, 2nd edition. Butterworh-Heineman, Boston, 1991. [ Links ]
5. GRAYDON, J.W. and KIRK, D.W. A microscopic study of the transformation of sphalerite particles during the roasting of zinc concentrate. Metallurgical Transactions B, vol.19B, 1988. pp. 141-146. [ Links ]
6. CHEN, T.T. and DUTRIZAC, J.E. Mineralogical changes occurring during the fluid-bed roasting of zinc sulphide concentrates. Journal of Metals, 2004. pp. 46-51. [ Links ]
7. DENOISEUX, R., WINAND, R., WILLEKENS, H., and VOS, L. Metallurgy Hoboken-Overpelt process for roasting zinc concentrates in a fluid bed. Proceedings of a World Symposium on Metallurgy and Environmental Control. Cigan, J.M., St. Joe Minerals Corporation, Monaca, Pennsylvania, Mackey, T.S., Key Metals and Minerals Engineering Corporation, Texas City, Texas, O'Keefe, T.J., and University of Missouri-Rolla, Rolla, Missouri. (eds.). Metallurgical Society of AIME, February 24-28, Las Vegas, Nevada, 1980. pp. 69-84. [ Links ]
8. BROWN, P., BOX, T., BROWNLEE, K., and GOLDSWORTHY, T. Pilot roasting of zinc concentrates. Lead & Zinc '95. Proceedings of the International Symposium on the Extraction and Applications of Zinc and Lead. Azakmi, T., Masuko, N., Dutrizac, J.E., and Ozberk, E. (eds). pp. 239-249. [ Links ]
9. BROWN, M.J. and GOOSEN, D.W. Fluidized-bed system for stabilized roasting of sulphide ore concentrates having controlled particle size. PCT International Appl. (1997) WO 9741268 A1. [ Links ]
10. CARREY, K.G. and HALL, J.S. Understanding the mechanism of dustless roasting in the fluidised bed calcination of fine grain zinc sulphides. Third World Congress of Particle Technology, Brighton, Victoria, July 1998. CSIRO Minerals, Clayton, Victoria Australia, 1997. pp. 1-8. [ Links ]
11. MCCARTHY, D.J., TAKOS, J., BOX, J., and BROWN, P.J. A process for producing agglomerates - A. Application for Canadian patent-2186312, 1994. pp. 1-12. [ Links ]
12. MCCARTHY, D.J., TAKOS, J., BOX, J., and BROWN, P.J. A process for producing agglomerates - B. Canadian Patent Application 2186312, 1994. pp. 1-9. [ Links ]
13. SANCHUAN, T., CHAO, B., and JIANE, S. Oxidation kinetics of marmatite and fluidization roasting of concentrate pellets. Proceedings of International Symposium on Extractive Metallurgy of Zinc, Tozawa, K, (ed.). Tokyo, Mining and Metallurgical Institute of Japan, Tokyo, Japan, 14-16 October, 1985. pp. 157-169. [ Links ]
14. KLUE, E.R. and DITTRICH, F. Oxygen enrichment program. Zincor Limited. Internal Report, 1985. pp. 1-17. [ Links ]
15. JORDAN, J.S. Freezing of roaster beds. Zincor Limited. Internal Report, 1986. pp. 1-5. [ Links ]
16. NATESAN, K. and PHILBROOK, W.O. Oxidation kinetic studies of zinc sulfide in a fluidized bed reactor. Metallurgical Transactions, May 1970. pp. 1353-1360. [ Links ]
17. CANNON, K.J. and DENBIGH, K.G. Studies on gas-solid reactions -I. Chemical Engineering Science, vol. 6, 1957. pp.145-159. [ Links ]
18. NATESAN, K. and PHILBROOK, W.O. Oxidation kinetic studies of zinc sulfide pellets. Transactions of the Metallurgical Society of AIME, vol. 245, October, 1969. pp. 2243-2250. [ Links ]
19. SIEMENS. Ultramat 6 and oxymat 6 analysers for IR-absorbing gases and oxygen. Manual 02.99. [ Links ]
20. SPIRA, P. A radioactive tracer test in No.1 roaster at Canadian Electrolytic. Noranda Research Centre. Internal report No.187. February 1970. [ Links ] ♦
Paper received Jun. 2009; revised paper received Jul. 2011.
© The Southern African Institute of Mining and Metallurgy, 2011. SA ISSN 0038-223X/3.00 + 0.00.














