Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.111 no.10 Johannesburg oct. 2011
TRANSACTION PAPER
Mineralogical solutions for pyrometallurgical problems
L. Andrews; P. den Hoed
Anglo American Research, Johannesburg, South Africa
SYNOPSIS
Mineralogy, traditionally associated with concentrator studies, is being increasingly applied to problems in pyrometallurgy. Such investigations commonly make use of a number of techniques, and the results are combined for a meaningful interpretation, which can lead to a successful solution for the problem.
Mineralogical techniques are described for various pyrometallurgical operations. Examples are given of studies and investigations involving mineralogy in chemical-looping combustion, feed monitoring to platinum-smelting furnaces, and losses to slag in base metal and platinum smelting.
Keywords: mineralogy, phase-chemical, chemical-looping combustion, XRD, SEM, EDS, QEMSCAN, PGM, slag, matte, ilmenite.
Introduction
Mineralogy can be thought of as an extra dimension in metallurgical planning and investigations, especially when combined with chemical or metallurgical information. Strictly defined as the study of minerals, including their distribution, identification, physical properties, and chemical composition, mineralogy is traditionally associated with the geosciences and concentrator studies, but can equally be applied to problems encountered in metallurgy1,2.
The most basic technique used in mineralogy is optical microscopy; in addition, modern phase characterization relies on the measurement of crystal structure and orientation by means of techniques such as X-ray diffractometry (XRD) and electronbackscattered diffractometry (EBSD), and the determination of phase shape, size, modal abundance, and composition with electronbeam techniques such as scanning electron microscopy and electron-probe micro-analysis. A mineralogical assessment will usually rely on a combination of several techniques.
Mineralogical applications in pyrometallurgy
Mineralogy can be used to characterize solid phases in furnace or roaster feeds and in the products of roasting, smelting, and converting. Some of the processes in which mineralogy can contribute are outlined below, and specific applications are described.
Roasting
Knowledge of the phase-chemical compositions and structures of roaster feed and products, and their variations over time, allows processes to be designed. The benefit of this knowledge can be seen, for example, when evaluating the performance of the oxygen carrier in chemical-looping combustion (CLC).
Application: chemical-looping combustion
CLC is a technology being developed in laboratories round the world. It addresses the matter of CO2 emissions to atmosphere by inherently separating the greenhouse gas during combustion3. A pure CO2 stream can be sequestrated. In a CLC process an oxygen carrier-an oxide, either natural or synthesized-is made to circulate between two fluidized-bed reactors, an 'air reactor' and a 'fuel reactor'. Fuel (e.g. natural gas or coal) is introduced to the fuel reactor, where it reduces the oxygen carrier and forms CO2 and H2O (Equation [1] describes the reaction in schematic form). Condensation separates H2O from CO2. The reduced oxygen carrier is then transferred to the air reactor where, in contact with air diluted by nitrogen, it is re-oxidized (Equation [2])3. Temperatures in the two units run at about 950ºC. Reaction 1 is often endothermic; reaction 2 is exothermic. Nevertheless, the total heat evolved from both reactions equals that released under normal combustion, where oxygen is in direct contact with the fuel. Thus, without losing energy, but achieving a separation of CO2 from N2, CLC has an emission advantage over normal combustion.

The matter that concerns us here, however, is the behaviour of the oxygen carrier. A first requirement is the selection of a good carrier. Carriers are required to exhibit high reactivity, good mechanical strength, and resistance to attrition and agglomeration. The carrier particles should also retain their integrity during many cycles of oxidation and reduction. Candidates have included oxides of iron, manganese, cobalt, nickel, and copper4,5. Ilmenite (FeTiO3) has become a popular oxygen carrier, because it is abundant and comparatively cheap. It has been tested for a number of years, now, but it has never been fully examined; its effectiveness has been determined by such measures as how much fuel it can convert and how quickly and, in doing so, what its oxygen-carrying capacity is. That is not to say that there have not been reports characterizing the oxygen carrier: phases have been identified, but their distribution in particles has not been examined. In recent years studies have published images of the internal structures of particles, but these have not been tied to the distribution of phases. Some of the images nevertheless point to the performance of the oxygen carrier over the long term. So, for example, 'ilmenite' particles after a number of cycles show an iron-enriched (oxide) rim on the surface of particles and an exfoliation shell6. The shell appears somewhat fragile; it is very likely that the mechanical forces in a fluidized bed will, in time, break this shell from the rest of the particle. Each subsequent shell will, in all likelihood, meet the same fate. This possibility warrants investigating.
The changes that iron-titanium oxides undergo during redox cycles in CLC can be evaluated in an informative manner by referring to the chemical thermodynamics of the oxide system. A useful tool in this regard is the equilibrium phase diagram, which is well documented for the Fe-Ti-O system. The phases and their relations described by the diagram can be compared with those observed in samples of actual oxygen carriers at different stages in the process. Using XRD, and by examining (polished) sections of particles under the electron microscope and measuring phase compositions with energy-dispersive X-ray spectroscopy (EDS), one can determine phase-chemical compositions in the samples and compare them with those described in the appropriate phase diagrams. A first step in this direction has been made with the application of phase relations in the system Fe-Ti-O to the performance of 'ilmenite' in CLC7. The chemical thermodynamics predict that FeTiO3 oxidizes to Fe2TiO5 (pseudobrookite) and TiO2 (rutile), a condition reflected by a move on the oxygen reaction line XY towards Y (see Figure 1). Subsequent reduction returns the oxide either to FeTiO3 (ilmenite) if the system is held at equilibrium or to Fe2TiO4 (ulvöspinel) if conditions prevent TiO2 (rutile) from taking part in the reduction. Which of the oxides actually forms, how much of it forms, and how it is distributed has yet to be confirmed. Once we know this, a comparison of predicted and observed outcomes will allow us to evaluate an aspect of the performance of the oxygen carrier: how close to the limit (where metallic iron is formed-an undesirable outcome) the oxygen carrier can be pushed in the fuel reactor, and therefore the extent to which the oxygen transport capacity of the carrier is diminished (if at all).
The oxygen transport capacity is defined as

where mox is the mass of a carrier in its oxidized state and mred is its mass in the reduced state. The higher the oxygen transport capacity, the less the oxygen carrier required for the job. The oxygen transport capacity of FeTiO3 (ilmenite) is 5.0%, which compares with 21.4% for Ni-NiO and 20.1% for Cu-CuO. If TiO2 (rutile), once formed, remains inert in subsequent cycles, then one can improve the oxygen transport capacity for iron-titanium oxides only slightly by synthesizing either Fe2TiO5 (pseudobrookite) or Fe2TiO4 (ulvöspinel). Ro rises to 6.7%, which remains a comparatively low value. Any diminished capacity, by not reducing or oxidizing the carrier to its full extent, only makes matters worse. Confirmation of the phases formed, their individual compositions, and distributions throughout the carrier particles will help identify the nature of any diminished capacity. It will provide an important piece in the puzzle and do much to establish the full potential of ilmenite as an oxygen carrier in chemical-looping combustion.
Smelting/converting
Feed
Several types of furnace feed (ore concentrates or flash-dryer products) may possess very similar chemical compositions, but display completely different melting characteristics. Accurate knowledge of the mineral content allows prediction of the energy requirements, the melting temperature, and the matte fall.
Application (furnace feed)
Concentrates (smelter feeds) produced from the Bushveld Complex in North West and Limpopo provinces by Anglo Platinum Ltd are routinely monitored by means of mineralogical and chemical analysis. Merensky Reef, UG-2, and Platreef final concentrates are sampled at the concentrators; flash-dryer feeds are sampled at the smelters9. Automated mineralogy techniques (QEMSCAN® or MLA) determine the mineralogy of the concentrates, specifically the modal percentage and type of gangue minerals and base metal sulfides (Figure 2). Mineralogical and compositional results may be combined to predict the smelting characteristics of the feed-parameters such as the matte fall, slag viscosity, and smelting energy requirements can be calculated by modelling. The information is databased so that trends can be identified. Feed concentrates can be blended to reduce potential problems, such as spinel formation in the furnace, variable sulfur levels, and the need for flux addition. These are dealt with in more detail below.

Many problems encountered in the furnaces are feedrelated. High levels of chromium in feed lead to chromium saturation of the slag (at Cr2O3 levels around 2% during normal furnace operation)10. This leads to crystallization of slag spinel ([Cr,Fe,Mg]3O4), which has a relatively high melting point. The spinel can form a layer between the slag and matte, one that hinders matte droplets in the slag from settling into the matte bath. Figure 3 shows the microtexture of a sounding-bar (dip) sample taken from a six-in-line furnace when spinel had formed just above the slag-matte interface. The semi-solid layer is more viscous than normal slag, and this causes problems for tapping and level control. Spoon samples from the slag taphole can also confirm the high spinel levels, but are not so easy to relate to depth in the furnace.

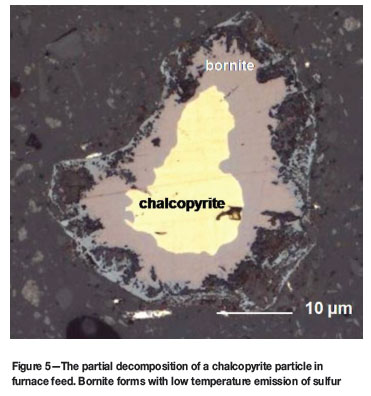
In sulfide smelting, a certain amount of sulfur in furnace feed facilitates matte fall and collection of value metals. Sulfide minerals in furnace feed melt to provide sulfur, but the amount of sulfur produced and the temperature at which it is released depend on the sulfide mineralogy of the feed. In a melting furnace, pyrite (FeS2) decomposes below the smelting temperature to produce FeS with a release of sulfur vapour (Figure 4). In a similar manner, chalcopyrite (CuFeS2) decomposes to produce FeS, Cu5FeS4 (bornite), and sulfur vapour (Figure 5). Cu5FeS4 can thermally decompose further to FeS and Cu2S, releasing additional sulfur vapour. These reactions can occur while the feed is situated over the slag bath as a 'black top', and can result in sulfur loss and sulfidation corrosion of various furnace structures.

The problems outlined above may be controlled to some extent by blending the final concentrates to balance the feed characteristics. Samples of flash-dryer feed (blended furnace feed) are taken from the belt and composited. Dried samples are later sent for chemical and QEMSCAN® analysis, and these results are reported to the smelters and included in the database.
Furnace/converter products-matte
Problems in processing or refining of furnace or converter mattes may be prevented by defining the mineralogy and composition of the matte after it has been cast or quenched. Studies of platinum converter mattes, for example, reveal that the mineralogy of constituent phases has a direct bearing on the choice of downstream recovery process, and is affected by even small changes in upstream (feed) metallurgy11,12. The phases which form in slow-cooled and quenched mattes are related not only to the cooling rate, but also to the major-element composition of the melt. If the matte is to be slow cooled and then concentrated by magnetic separation, the size, shape, and magnetic susceptibility of the PGE-bearing alloy phases has to be optimized, and this is done by controlling iron and sulfur levels for a given nickel-to-copper ratio.
Furnace/converter products-slag
It is essential we understand the type of losses to a furnace or converter slag if the slag is to be treated further or cleaned successfully. In base metal and platinum smelting, values can be trapped in slag as mechanically entrained matte or metal, or chemically bound in oxides or silicates. The size and relative amounts of the phases involved can be inferred from the mineralogy, and the most suitable cleaning process can be used. Take, for example, relatively large particles of matte or metal entrained in the slag and low solubilities of base metals in these slag silicates. The entrained particles bearing the base metals can be recovered by milling and flotation. Fine matte- or metal-size distributions and high base-metal solubilities in silicates, on the other hand, would require pyrometallurgical slag cleaning in a slag-cleaning furnace. The use of reductant and suitable settling times would aid recovery.
Application-slag losses
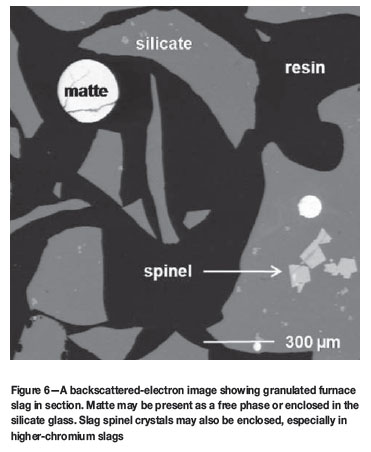
Losses of PGEs and base metals (nickel, copper, and cobalt) to slags from the six-in-line and slag-cleaning furnaces of Anglo Platinum Ltd are monitored regularly at Anglo American Research. The smelting parameters that affect and control base-metal losses are numerous13; they include slag composition, basicity ratio, smelting temperature, and oxygen potential. The factor found to be the most important in determining base-metal dissolution into furnace slag at Anglo Platinum Ltd is the degree of oxidation, or slag pO2. Prior to 2001, converter slag was returned regularly to the six-in-line furnaces. This raised the furnace-slag pO2 and gave rise to higher base-metal dissolution in, and therefore losses to, the slag. A study of slag samples taken five years apart combined techniques, such as chemical analysis (for bulk slag composition), electron-probe microanalysis (to measure small amounts of dissolved, or chemically-bound, base metals in slag), scanning electron microscopy (to examine mechanically entrained matte droplets in slag), and Mössbauer Spectroscopy (to measure slag Fe3+/Fe2+ and hence calculate pO2)14,15. Images of quenched furnace slag and the instrument currently used for the microanalysis of dissolved base metals are shown in Figures 6 and 7. Base metals are concentrated in the entrained matte sulfides. The amounts of these metals dissolved in slag silicate glass are much lower (Table I), but the high ratio of silicate to entrained matte, combined with the small size of entrained matte inclusions, leads to more base metals being lost in discarded silicate than are recovered by sulfide flotation (Table II).


As well as demonstrating the higher dissolved base-metal losses introduced by converter slag return, the investigation showed that tapped slag is not in equilibrium with bulk matte. One aspect of this is that modelling software underes-timates base-metal losses to slag, because equilibrium is usually assumed. It is likely that the same applies to basemetal smelting.
Other applications
Mineralogy is helpful in many other areas of pyrometallurgy, such as flux or furnace additive and off-gas solids characterization, and refractory failure and compatibility investigations.
Conclusions
Mineralogy and the techniques in mineral phase analysis are being applied in a wide variety of pyrometallurgical investigations, where they assist in process design, problemsolving, and model validation. The paper considers four examples:
The performance of FeTiO3 (ilmenite) as an oxygen carrier in chemical-looping combustion. The phase chemistry of the Fe-Ti-O system shows that FeTi2O5 (pseudobrookite) and TiO2 (rutile) are formed during oxidation; that subsequent reduction forms either FeTiO3 (ilmenite) or Fe2TiO4 (ulvöspinel), depending on whether the TiO2 (rutile) formed takes part in further reaction or not. Identifying phases, their compositions, and morphologies by means of XRD, SEM, and EDS will confirm which course the redox reactions follow, and in turn answer questions about the performance and potential of ilmenite as an oxygen carrier
Problems related to the phase-chemical composition of furnace feed to the smelters at Anglo Platinum Ltd can be alleviated by blending according to concentrate composition and mineralogy
Difficulties encountered in the processing of furnace and converter mattes may be avoided by careful control of matte composition and monitoring of the product mineralogy
The losses of PGEs and base metals to furnace slag at Anglo Platinum Ltd have been characterized, and it has been established that current modelling practises must be validated by mineralogical analysis.
Acknowledgements
This paper is published by permission of Anglo American Research and Anglo Platinum Ltd. The contributions of Lloyd Nelson and Rodney Hundermark (Anglo Platinum Ltd.) are gratefully acknowledged.
References
1. DE VILLIERS, J.P.R. and VERRYN, S. Modern techniques in X-ray diffraction applied to metallurgy, Journal of the Southern African Institute of Mining and Metallurgy, vol. 107, no. 2, 2007. pp. 83-86. [ Links ]
2. ANDREWS, L. The use of electron microbeam techniques in metallurgical analysis, Journal of the Southern African Institute of Mining and Metallurgy, vol. 107, no. 2, 2007. pp. 79-82. [ Links ]
3. Lyngfelt, A., Johansson, M., and Mattisson, T. Chemical-looping combustion-Status of development. CFB9, 9th International Conference on Circulating Fluidized Beds, Hamburg University of Technology, Germany, 2008. pp. 39-53. [ Links ]
4. ADÁNEZ, J., DE DIEGO, L.F., GARCÍA-LABIANO, F., GAYÁN, P., and ABAD, A. Selection of oxygen carriers for chemical-looping combustion. Energy Fuels, vol. 18, 2004. pp. 371-377. [ Links ]
5. ADÁNEZ, J. Oxygen carrier materials for chemical-looping processes- Fundamentals, 1st International Conference on Chemical Looping. Lyon, Les Rencontres Scientifique de l'IFP, 2010. [ Links ]
6. Adánez, J., Cuadrat, A., Abad, A., Gayán, P., De Diego, L.F., and García-Labiano, F. Ilmenite activation during consecutive redox cycles in chemical-looping combustion. Energy Fuels, vol. 24, 2010. pp. 1402-1413. [ Links ]
7. DEN HOED, P. and LUCKOS, A. The oxidation and reduction of iron-titanium oxides in chemical-looping combustion: A phase-chemical description. Oil and Gas Science and Technology. Revue IFP Energies Nouvelles, vol. 66, no. 2, 2011. pp. 249-263. [ Links ]
8. Den Hoed, P. and Luckos, A. The oxidation and reduction of ilmenite in chemical-looping combustion: A phase-chemical description, 1st International Conference on Chemical Looping, Lyon, Les Rencontres Scientifique de l'IFP, 2010. [ Links ]
9. MKHIZE, B. and ANDREWS, L. Smelter feed quality control. Minerals Engineering, In press. [ Links ]
10. NELL, J. Melting of platinum group metal concentrates in South Africa, Journal of the Southern African Institute of Mining and Metallurgy, vol. 104, no. 9, 2004. pp. 423-428. [ Links ]
11. SCHOUWSTRA, R.P. The slow-cooling process: The impact of composition on the quality and subsequent processing of converter matte-a MRM perspective. M.Sc. dissertation, University of the Free State, South Africa. 2003. [ Links ]
12. THYSE, E., AKDOGAN, G., and EKSTEEN, J.J. The effect of changes in ironendpoint during Peirce-Smith converting on PGE-containing nickel converter matte mineralization. Minerals Engineering, vol. 24, no. 7, 2011. pp. 688-697. [ Links ]
13. ERIC, R.H. Slag properties and design issues pertinent to matte smelting electric furnaces, Journal of the Southern African Institute of Mining and Metallurgy, vol. 104, no. 9, 2004. pp. 499-510. [ Links ]
14. ANDREWS, L., PISTORIUS, P.C., and WAANDERS, F.B. Electron beam and Mossbauer techniques combined to optimise base metal partitioning in the furnace, Microchimica Acta, vol. 161, 2008. pp. 445-450. [ Links ]
15. ANDREWS, L. and PISTORIUS, P.C. Nickel, copper and cobalt distribution and equilibria in Anglo Platinum furnace slags, Transaction of the Institute of Mining and Metallurgy Section C, vol. 119, no. 2, 2010. pp. 52-59. [ Links ] 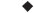
This paper was first presented at the Southern African Pyrometallurgy Conference, 6-9 March 2011, Misty Hills, Muldersdrift.
© The Southern African Institute of Mining and Metallurgy, 2011. SA ISSN 0038-223X/3.00 + 0.00.














