Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.111 no.10 Johannesburg oct. 2011
TRANSACTION PAPER
ISACONVERTTM-continuous converting of nickel/PGM mattes
M.L. Bakker; S. Nikolic; A.S. Burrows; G.R.F. Alvear
Xstrata Technology, Brisbane, Australia
SYNOPSIS
The ISASMELTTM top submerged lance (TSL) bath smelting process was developed in Mount Isa, Australia by Mount Isa Mines Limited (now a subsidiary of Xstrata plc) during the 1980s. By the end of 2011, the total installed capacity of the ISASMELTTM technology will exceed 8 000 000 metric tons per year of feed materials in copper and lead smelters around the world. Commercial plants, operating in Belgium and Germany, are also batch converting copper materials in ISASMELTTM furnaces. This TSL technology is equally effective for continuous converting processes, in which role it is called ISACONVERTTM.
This paper presents the recently patented ISACONVERTTM process for the continuous converting of nickel and platinum group metal (PGM) containing mattes using the calcium ferrite slag system. The paper focuses on the potential application of the ISACONVERTTM technology to existing nickel and PGM smelting complexes.
Keywords: pyrometallurgy, top submerged lance (TSL), nickel, PGM, calcium ferrite, lime ferrite.
Introduction
ISASMELTTM top submerged lance (TSL) technology is well established as one of the standard technologies for primary copper smelting1. Over the years, ISASMELTTM has progressed from a 0.25 t/h pilot plant scale to industrial facilities that process up to 200 t/h feed. The technology can treat a variety of materials including nickel2, lead3, and copper4concentrates and secondary materials, with the total installed capacity of ISASMELTTM plants expected to exceed 8 000 000 t/a by the end of 2011.
Batch converting in the ISASMELTTM furnace has been performed by two smelters in Europe, namely Umicore Precious Metals in Hoboken, Belgium5, and Aurubis AG, Lünen, Germany6, since 1997 and 2002 respectively. The process has also been adapted to continuous converting of copper matte to blister copper7-9 and low-grade nickel matte to Bessemer matte2,10, in which role it is called the ISACONVERTTM process.
Development of the Nickel/PGM ISACONVERTTM process
The ISACONVERTTM process shares many design features with the ISASMELTTM furnace6. It can readily be enclosed to minimize emissions to the surrounding environment. It uses the TSL injection technology to provide highly efficient mixing and reaction of solid matte and flux, which can be charged through the roof of the furnace. The use of advanced process control systems results in the furnace operation being largely automated. Being a vertical furnace, very little floor space is required to accommodate the plant and so it can generally be easily retrofitted into existing smelting facilities to either augment or replace existing technology. The significantly reduced off-gas volume from the ISACONVERTTM process, when compared to Peirce-Smith technology, results in lower capital and operating costs for off-gas collection and cleaning systems6.
This technology has now been applied by Xstrata Technology (XT) for the continuous converting of low-grade nickel/PGM matte to high-grade Bessemer matte - the patented Nickel ISACONVERTTM process. Analogous to the ISACONVERTTM process for copper, the Nickel ISACONVERTTM process also employs the calcium ferrite slag system.
Nickel/PGM ISACONVERTTM process concept
The feed to a PGM smelter typically consists of nickel-copper sulfides and refractory oxide materials11. The product from PGM smelters is generally a high-iron containing smelter matte which is further processed, almost exclusively using multiple units of Peirce-Smith converters, to produce finished, lowiron containing matte, often referred to as 'Bessemer matte'. The exception is the Anglo Platinum Waterval smelter in South Africa, where the Anglo Platinum Converting Process (ACP) is employed12.
Continuous PGM matte converting is not a new concept, and has been investigated previously for improving productivity and emission control compared to the traditional Peirce-Smith batch converters. As noted above, the ACP plant has already commercialized this process concept.
XT has investigated nickel/PGM matte converting with the ISACONVERTTM technology2,8,13,14 and produced mattes containing less than 4 wt% iron successfully on the pilot scale. The ISACONVERTTM process for treating PGM matte is a continuous converting process with matte and air/oxygen fed continuously to the bath. The bath consists of matte and slag at the product compositions at all times. The operating conditions effectively fix the process at what is, for Peirce-Smith converters, the end-point of the converting reactions.
Figure 1 shows how the ISACONVERTTM process could be integrated into the flowsheet of an existing smelting facility to treat PGM matte. Granulated smelting furnace matte feed, limestone flux, purchased feed, furnace dusts, fuel, air, and oxygen would be fed continuously to the ISACONVERTTM furnace. The product liquid, low-iron Bessemer matte would be tapped periodically from the matte taphole and, depending on downstream refinery requirements, the matte may be either granulated or slow-cooled.
Slag would be tapped through a separate taphole and returned to the primary smelting plant for recovery of the metal values. Off-gases from the ISACONVERTTM furnace would be directed to a waste-heat boiler for energy recovery, and de-dusted using an electro-static precipitator before being sent to either an existing gas treatment facility or to a dedicated sulfuric acid plant for sulfur capture. All dusts collected from the gas handling systems would be recycled to the ISACONVERTTM furnace.
The ISACONVERTTM process presented in Figure 1 offers two principal advantages compared with traditional batch Peirce-Smith converting:
Firstly, the ISACONVERTTM process generates a constant volumetric flow rate of off-gas, containing a high level of SO2 that can readily be treated in a conventional sulfuric acid plant. This is an important benefit, considering the stringent environmental regulations affecting both current and future plant emissions and in-plant hygiene. While fitting tight converter hoods remains a potential option to capture Peirce-Smith converter off-gas, this approach, coupled with the additional need for secondary hooding to control fugitive emissions, is typically a high-cost option. ISACONVERTTM offers a one-step, one-furnace converting process that can utilize high levels of oxygen enrichment coupled with minimal air dilution.
Secondly, the ISACONVERTTM process offers the use of solid matte as the feed material, thus eliminating molten matte ladle transfers, and further reducing the potential for fugitive emissions, with a resulting improvement in plant hygiene. The use of solid feed also allows decoupling of the smelting and converting steps, giving added flexibility and simplifying the maintenance and operational aspects of the smelter.
Continuous converting process slag chemistry
Current PGM matte converting slag chemistry
Both the batch Peirce-Smith converter and the continuous ACP nickel matte converting processes use an iron silicate (fayalite)-based slag system. Peirce-Smith converting furnaces typically convert molten primary smelting furnace matte to a final matte product containing 1-3 wt% iron. Rapid precipitation of magnetite (predominately nickel-ferrite) in slag restricts Peirce-Smith converters to an end point of approximately 2 wt% iron in matte. Some operators solidify the remaining slag within the Peirce-Smith converter vessel before continuing the blowing cycle, to lower iron in matte levels15. The practice of solidification during final blowing generates a mush of silica and magnetite-saturated slag that holds within it final product matte that can be recovered only through the start of a new converting cycle15, generating process inefficiencies. Operation that is continued below 2 wt% iron in matte, without solidification of the slag, results in either excessive magnetite/slag entrainment within the product matte or increased furnace build-up15.
The original flowsheet for the ACP involved two-stage batch production of Bessemer matte: a first stage to lower the iron in matte content to ~13 wt%, and the second stage to lower it to ~3 wt% iron in matte12. Due to difficulties associated with determining starting points for the second stage of converting, slag eruptions occurred due to overoxidation of the bath12. The batch nature of the process resulted in poor or incomplete mixing, which led to nonequilibrium stratification of the melts within the furnace. Subsequent rapid mixing of the melt layers due to bath perturbations resulted in explosive foaming of the bath contents at low iron-in-matte levels12. For these reasons, and to maintain a constant high-strength SO2 gas stream to the off-gas processing facility, the ACP was modified to a continuous process, with granulated matte continuously fed to the furnace and converting at an end-point of 3 wt% iron in matte12.
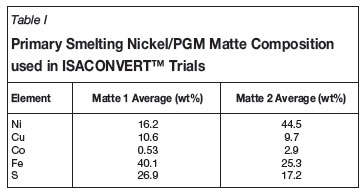
Preliminary pilot-plant test work campaigns for the ISACONVERTTM process used iron silicate- based slags for treating two different matte feeds, as shown in Table I. Final mattes produced contained between 0.7-13 wt% iron from Matte 1 feed14and 2.2-10 wt% iron from Matte 2 feed2,8. The results of the iron silicate slag converting test work campaigns highlighted that production of mattes containing less than 2 wt% iron required the temperature of the process to be increased substantially to maintain fluidity of the iron silicate slag. XT therefore investigated the applicability of a calcium flux- based slag system, as used successfully in the copper ISACONVERTTM process.
ISACONVERTTM Nickel/PGM converting slag chemistry
The calcium ferrite slag system has been successfully applied to continuous copper converting technologies since the mid1970s16. The beneficial properties of calcium ferrite slags for copper converting were established and outlined in the 1980s by the research of Yazawa17-18 and Takeda19. These include the ability of the liquid phase to contain higher ferric iron concentrations at high oxygen potentials, lower slag volume, lower valuable metal losses, and greater fluidity.
These beneficial properties were investigated and confirmed by the laboratory-scale research work of Font20and Henao21, indicating higher valuable metal recovery (Ni, Cu, and Co-which carry PGM components) while also increasing the distribution of impurity elements (As and Sb) to the slag. XT used the thermodynamic modelling package FactSage22to confirm that the beneficial properties of the calcium ferrite system, in terms of fluidity and ferric iron capacity, also applied to the process of converting nickel/PGM mattes.
Nickel matte converting using calcium ferrite slag at the commercial scale has been successfully applied by Stillwater Mining Company (SMC) in batch top blown rotary converters (TBRCs), producing a Bessemer matte containing about 2 wt% iron11since 1991. The TBRC process at SMC originally used an iron-silicate based slag for converting, but experienced sudden slag foaming at low iron-in-matte levels, from over-oxidation, causing loss of charge and potential threats to the safety of the equipment and the operators23. SMC consequently altered their process slag chemistry by adding lime-based flux instead of silica, thereby avoiding the formation of unstable bath conditions.
Considering the successful application of calcium ferrite slags to batch nickel matte converting at SMC, and the proven ability of the ISACONVERTTM and other processes to use calcium ferrite slags for copper production5-7,16, the application of this slag system to a continuous TSL process for nickel/PGM matte production was investigated by XT through pilot-scale test work.
The objective of the pilot-scale converting test work was to confirm and demonstrate the nickel/PGM matte ISACONVERTTM process chemistry for converting high-iron primary smelting matte feed to low-iron Bessemer matte, utilizing a calcium ferrite-based slag. The tests involved converting solid matte (refer to Matte 2 composition in Table I), with the addition of limestone flux to the pilot furnace at a rate of 100-150 kg/h of 'as received' solid matte. The details of the ISACONVERTTM pilot-plant facility have been published elsewhere8. The pilot-plant tests revealed that fluid slags were produced under all test conditions.
ISACONVERTTM test work results
The target grade for nickel/PGM Bessemer matte is typically matched to the requirements of the downstream refinery that specifies the permissible level of iron and sulfur within the matte. A comparison of the matte grade (with respect to the concentration of iron the matte), between the ISACONVERTTM calcium ferrite process test work, Peirce-Smith converters, and a TSL using iron silicate slag is shown in Figure 2. Sources for the Peirce-Smith converting and TSL iron silicate slag data included:
Results from TSL iron silicate slag-matte converting pilot plant tests conducted by XT2,8
Results from a sampling campaign of a Peirce-Smith converter blow at:
- The Xstrata Nickel Falconbridge smelter24and
- The Vale Inco Thompson smelter25.
At a fixed iron-in-matte concentration, the ISACONVERTTM calcium ferrite process produces a matte grade that is over five mass per cent richer in nickel, copper, and cobalt compared to converting with an iron silicate slag process. The increased Bessemer matte grade achieved using calcium ferrite slags is a consequence of lower sulfur concentrations in the matte phase, when compared to iron silicate slags. This is displayed graphically in Figure 3, where the sulfur deficiency the matte (defined in Equation [1] of reference26) is compared to the iron concentration in the matte.
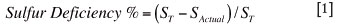
where:
ST = sulfur required to form sulfide phases Ni3S2, Cu2S, Co9S8, and FeS;
SActual = measured sulfur concentration the matte phase.
Feed matte to a nickel/PGM converting process is typically sulfur deficient; refer to Table I. Figure 3 shows that iron silicate nickel/PGM matte converting processes generate matte that is between 20-30% sulfur-deficient. Even after nitrogen-induced slow cooling, as practised by Vale Inco, sulfur deficiency is normally maximized at approximately 35%27. The ISACONVERTTM calcium ferrite slag process, however, allows for the production of a final product matte with an over 45% level of sulfur deficiency.
Mineralogical analysis of Peirce-Smith converter matte has revealed that metallized phases with the Bessemer matte are nickel-dominant and act as collectors for PGMs28. XT therefore postulates that the increased metallization of the ISACONVERTTM process, (Figure 3) would also result in increased concentration and deportment of PGMs to the final product Bessemer matte, when compared to Peirce-Smith converting or TSL iron silicate slag processes. Further pilotplant test work will be required to confirm these PGM deportments.
Conclusions
ISASMELTTM TSL technology is well established for both primary and secondary copper and lead smelting. Batch smelting and converting using ISASMELTTM technology is also well established. The technology is equally effective for continuous converting processes, in which role it is called ISACONVERTTM.
The features that make ISACONVERTTM attractive for nickel/PGM converting operations are:
1. Generation of a constant volumetric flowrate of off-gas containing a high level of SO2 that can be treated in a conventional sulfuric acid plant
2. A one-step, one-furnace converting process that can utilize high levels of oxygen enrichment, coupled with minimal air dilution and
3. Solid matte can be used as the feed material:
a. Eliminating molten matte ladle transfers, further reducing the potential for fugitive emissions and
b. Allowing for decoupling of the smelting, and converting steps, increasing flexibility, and simplifying maintenance and operational aspects of the smelter.
The use of the ISACONVERTTM process for primary smelting furnace matte converting has been successfully demonstrated on the pilot scale. Results have shown that, when compared to iron silicate slag Peirce-Smith and TSL processes, the ISACONVERTTM calcium ferrite process:
1. Produces a higher-grade matte (nickel, copper, and cobalt) at iron-in-matte levels corresponding to Bessemer matte production
2. Produces a lower sulfur, higher metallized content matte product.
Acknowledgements
The authors thank Xstrata Technology for permission to publish this paper.
References
1. DIAZ, C. Copper sulfide smelting: past achievements and current challenges, Proceedings of Copper 2010, Hamburg, Germany, June 2010. GDMB, Clausthal-Zellerfeld, vol. 7, 2010. pp. 2543-62. [ Links ]
2. BAKKER, M.L., ALVEAR, G.R., and KREUH, F.M. ISASMELTTM TLS-making a splash for nickel. Pyrometallurgy of Nickel and Cobalt2009. Proceedings of the International Symposium: The 48th Annual Conference of Metallurgists, Sudbury, Ontario, 23-26 August 2009. J. Liu, J. Peacey, M. Barati, S. Kashani-Nejad, and B. Davis, (eds.). Canadian Institute of Mining, Metallurgy and Petroleum, 2009. pp. 181-194. [ Links ]
3. ERRINGTON, B., ARTHUR, P., WANG, J., and DONG, Y. The ISA-YMG lead smelting process. Proceedings of the International Symposium on Lead and Zinc Processing, Kyoto, Japan, T. Fujisawa et al., (eds.), The Mining and Materials Processing Institute of Japan, 2005. pp. 581-599. [ Links ]
4. ARTHUR, P.S. and HUNT, S.P. ISASMELTTM-25 Years of continuous evolution. Floyd International Symposium on Sustainable Development in Metals Processing, M. Nilmani and W.J. Rankin, (eds.), NCS Associates, Australia, 2005. pp. 73-94. [ Links ]
5. VANBELLEN, F. and CHINTINNE, M. The precious art of metals recycling. Advanced Processing of Metals and Materials. F. Kongoli and R.G. Reddy, (eds.). TMS, Warrendale, Pennsylvania, vol. 1, 2006. pp. 43-52. [ Links ]
6. SCHMIDT, S. Aurubis AG-Hüttenwerke Kayser. unpublished research, March 2006. [ Links ]
7. EDWARDS, J.S. and JAHANSHAHI, S. Copper Converting, US Patent, No. 5,888,270, 30 March 1999. [ Links ]
8. EDWARDS, J.S. and ALVEAR, G.R.F. Converting using ISASMELTTM technology. Copper'07, the 6th Copper/Cobre Conference. Volume 3-The Carlos Diaz Symposium on Pyrometallurgy, Book 2. A.E.M. Warner, C.J. Newman, A. Vahed, D.B. George, P.J. Mackey, and A. Warczok, (eds). The Metallurgical Society of CIM, Toronto, 2001. pp. 17-28. [ Links ]
9. NIKOLIC, S., EDWARDS, J.S., BURROWS, A.S., and ALVEAR, G.R.F. ISACONVERTTM-TSL Continuous Copper Converting Update. International Peirce-Smith Converting Centennial: TMS 2009 Annual Meeting and Exhibition, J. Kapusta and T. Warner, (eds.), The Minerals, Metals & Materials Society, Warrendale, USA, 2009. pp. 407-414. [ Links ]
10. BAKKER, M.L. NIKOLIC, S., and MACKEY, P.J. ISASMELTTM TSL applications for nickel, Minerals Engineering, vol. 24, no. 7, 2011. pp. 610-619. [ Links ]
11. WARNER, A.E.M., DIAZ, C.M., DALVI, A.D., MACKEY, P.J., TARASOV, A.V., and JONES, R.T. JOM world nonferrous smelter survey. Part IV: Nickel: Sulfide, JOM, vol. 59, no. 4, 2007. pp. 58-72. [ Links ]
12. VIVIERS, P. and HINES, K. The new Anglo Platinum converting project, First Extractive Metallurgy Operators' Conference. AusIMM, Brisbane, 2005. pp. 101-108. [ Links ]
13. BARTSCH, P., ANSELMI, B., and FOUNTAIN, C.R. The Radio Hill Project, Pyrosem WA. E.J. Grimsey and N.D. Stockton, (eds.), Perth, Murdoch University Press, 1990. [ Links ]
14. PGM matte converting, Xstrata Technology, Internal Report, 1996. [ Links ]
15. BEZUIDENHOUT, G.A., EKSTEEN, J.J., WENDT, W., and PERSSON, W. Implementation of Semtech optical spectrometry system at Lonmin for converter Fe end-point control, Nickel Processing 2010. Minerals Engineering International, Falmouth, UK, 2010. [ Links ]
16. DAVENPORT, W.G.L. et al., Extractive Metallurgy of Copper. 4th edn., Pergamon, Oxford, United Kingdom, 2002. [ Links ]
17. YAZAWA, A., TAKEDA, Y., and WASEDA, Y. Thermodynamic properties and structure of ferrite slags and their process implications, Canadian Metallurgical Quarterly, vol. 20, 1981. pp. 129-34. [ Links ]
18. YAZAWA, A. and TAKEDA, Y. Equilibrium relations between liquid copper and calcium-ferrite slag. Transactions of the Japan. Institute of Metals, vol. 23, 1982. pp. 328-33. [ Links ]
19. TAKEDA, Y., NAKAZAWA, S., and YAZAWA, A. Thermodynamics of calciumferrite slags at 1200 and 1300ºC, Canadian Metallurgical Quarterly, vol. 19, 1980. pp. 297-305. [ Links ]
20. FONT, J.M. Phase equilibrium and minor elements distribution between slag and matte phases in nickel smelting. PhD thesis, Tohoku University, 1999. pp. 63 and 66. [ Links ]
21. HENAO, H.M.Z. Phase equilibrium between Ni-S or Ni-Fe melt and slag in nickel smelting. PhD thesis, Tohoku University, 2003. pp. 54 and 60. [ Links ]
22. BALE, C.W. PELTON, A.D., and THOMPSON, W.T. Facility for the analysis of Chemical Thermodynamics (FactSage version 6.1). Ecole Polytechnique de Montreal, 2002. [ Links ]
23. ROSET, G.K., MATOUSEK, J.W., and MARCANTONIO, P.J. Converting practices at the Stillwater Precious Metals Smelter. JOM, April 1992. pp. 39-42. [ Links ]
24. BUSTOS, A.A., IP, S.W., O'CONNELL, G., KAIURA, G.H., and TOGURI, J.M. Converting Simulation at Falconbridge Limited, Extractive Metallurgy of Nickel and Cobalt. G.P. Tyroler and C.A. Landolt, (eds.). The Metallurgical Society, Inc, AIME, USA, 1988. pp. 335-354. [ Links ]
25. DIAKOW, J.S., MAK, Y.F., and ORR, R.G. Metallurgy of the converting process in the Thompson Smelter. 14th Annual Conference of Metallurgists, Edmonton, Alberta, August 1975, Montreal, Quebec. Canadian Institute of Mining, Metallurgy and Petroleum, 1975. [ Links ]
26. KELLOGG, H.H. Thermochemistry of nickel-matte converting. Nickel Metallurgy, vol. I: Extraction and Refining of Nickel, 25th Annual Conference of Metallurgists. E. Ozberk and S.W. Marcuson, (eds.). Canadian Institute of Mining, Metallurgy and Petroleum, Montreal, Canada, 1986. pp. 95-128. [ Links ]
27. WARNER, A.E.M. and DIAZ, C.M. An overview of the metallurgy of nickelcopper matte converting. Metallurgical and Materials Processing: Principles and Technologies (Yazawa International Symposium), vol. 2, High-Temperature Metal Production. H.Y. Sohn, K. Itagaki, C. Yamauchi, and F. Kongoli, (eds.). Warrendale, The Minerals, Metals & Materials Society, 2003. pp. 113-129. [ Links ]
28. THYSE, E., AKDOGAN, G., and EKSTEEN, J.J. The effect of changes in ironendpoint during Peirce-Smith converting on PGE-containing nickel converter matte mineralization, Nickel Processing 2010 Minerals Engineering International, Falmouth, UK, 2010. [ Links ] 
This paper was first presented at the Southern African Pyrometallurgy Conference, 6-9 March 2011, Misty Hills, Muldersdrift.
© The Southern African Institute of Mining and Metallurgy, 2011. SA ISSN 0038-223X/3.00 + 0.00.














