Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.111 n.6 Johannesburg Jun. 2011
JOURNAL PAPERS
Reducing water consumption at Skorpion Zinc
H. Bhikha; A.E. Lewis; D.A. Deglon
Minerals to Metals Research Initiative, Department of Chemical Engineering, University of Cape Town, Cape Town, South Africa
SYNOPSIS
The minerals industry is committed to the principles of sustainability. Reducing water consumption is a priority area, especially for regions of water scarcity. This paper presents a systemic optimization of the water balance of the Skorpion Zinc refinery with the aim of reducing water consumption. An Aspen Plus simulation of the process is used. The validity of the simulation is tested by measuring key output variables and comparing results to plant data. A number of water minimization scenarios are investigated, including unit operation and circuit configuration changes. The scenarios leading to the largest reduction in water consumption are through the full recycle of treated effluent water, which results in water savings of up to 19%. Reducing process water and/or recycling of untreated water is prohibited by the build-up of trace elements, which affect product purity. The Skorpion process already features a highly optimized water balance, with unit operational changes merely resulting in a shift in the water balance. Consequently, the largest area for improvement is through the reuse of effluent water.
Keywords: Skorpion zinc water usage, process optimization, sustainability.
Introduction
The Skorpion Zinc refinery, located in Rosh Pinah in southern Namibia, is unique due to its unconventional orebody and the use of solvent extraction, which is a revolutionary departure from conventional zinc processes1. The Skorpion Zinc process produces high quality zinc at relatively low operating costs. It produces a 99.995% Special High Grade (SHG) zinc product2, using a zinc oxide concentrate due to the oxidized ore reserves present in the region. This means that the concentrate cannot be treated via conventional zinc processing methods, which are based on a zinc sulphide concentrate feed.
The mining industry has historically been associated with many environmental issues. The industry is increasingly committed to maximizing the contribution of the mining and minerals sector to sustainable development3 . Sustainability has become a guiding factor in process design and implementation, rather than a passing thought. This is especially important since the mining industry consumes large amounts of energy and water and produces large volumes of waste. Water is probably the most widely used raw material in the process industry4 . There is significant pressure on industry to reduce water consumption due to the scarcity of good quality industrial-grade water, and the increase in water costs. Due to these water issues, correlations are being developed to determine the level to which water minimization is possible for a given process. One such example is that proposed by Norgate and Lovel5 .

where
| W | = | 'cradle to grave' water consumption (m3/ton refined metal) |
| G | = | grade of ore (% metal) |
Due to its location in an environmentally sensitive and arid region, water minimization is particularly important for Skorpion Zinc. The zinc industry in general has to cope with increasing environmental pressure, due to the environmental and social demands placed by the metallurgical industry6.
The objective of this paper is to systemically optimize the water balance of the Skorpion Zinc refinery with the aim of reducing water consumption. An Aspen Plus simulation of the process is used. The validity of the simulation is tested by measuring key output variables and comparing the results to plant data. The simulation process is critical, given that water quality affects key threshold concentrations throughout the process, most significantly the concentrations of Mn2+, Mg2+ and Cl-. The concentration of these trace impurities, if the thresholds are exceeded, impacts adversely on the final zinc product purity i.e. knowledge of trace concentrations is essential. A number of water minimization scenarios are investigated, including process variable changes and circuit configuration changes, informed by process design heuristics.
Research methodology
Process description and flow sheet
A simplified process flow sheet is given in Figure 1. The ore initially undergoes a three-stage crushing process following its extraction from the mine, where it is reduced to a size suitable for leaching. The crushed ore is then leached at atmospheric pressure with sulphuric acid to form a sulphate solution. This solution, known as the pregnant leach solution (PLS), then undergoes neutralization, where certain impurities such as iron, aluminium and silica are precipitated from solution. Any zinc that is precipitated in neutralization is recovered in the basic zinc sulphate (BZS) precipitation plant.
The clarified PLS proceeds to the solvent extraction step, where it is purified using the organic solvent D2EHPA (di(2-ethylhexyl) phosphoric acid). The raffinate is recycled back to the leaching step and any metals that co-extract with zinc are removed via cementation with zinc dust. The purified and upgraded solvent extraction product is then sent to electrowinning for the recovery of the SHG product.
Process simulation
The aim of the simulation was to obtain a working mass balance of the operation, from which different water minimization scenarios could be investigated. As noted previously, the simulation process was critical as water quality affects key threshold concentrations which, if exceeded, affect final zinc product purity. Due to the complexity of the Skorpion process, with numerous recycles and variables, a number of simulations were performed. Each simulation increased in complexity until a final working simulation was obtained that modelled the operating conditions of the plant as closely as possible. The simulation package used was Aspen Plus, for which the applicability for process simulation is well documented7-9. A summary of the simulation is presented below, with only the major reactions shown.
Key process operations and reactions
The main zinc-bearing mineral in the ore is sauconite (Na0.33Zn3(Si,Al)4O10(OH)2.4H2O), with smithsonite (ZnCO3 ) and hemimorphite (Zn4Si2O7(OH)2.H2O) occurring to a lesser degree. All reactions were carried out at a fixed conversion, since kinetics data was not available. The main zinc reactions in the leaching step were formulated as follows:
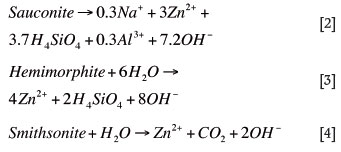
These reactions were fixed at a conversion of 95% based on actual plant data. The remaining minerals occurring in the ore were considered to be oxides, since the orebody itself is an oxidized ore. The built-in electrolyte chemistry in Aspen was used to obtain equilibrium concentrations of ions based on operating conditions. This was essential due to the impact of trace impurities on final zinc product purity.
In the neutralization step, Fe3+ and Al3+ were precipitated out of solution as hydroxides, using a limestone slurry. The main thickener in the process was modelled as a perfect solid-liquid separator, with an underflow solids content comparable to the plant. In the reacidification step, precipitated zinc was placed back into solution through the addition of H2SO4, which was used to control the pH. In the washing and filtration step, the efficiency was adjusted to achieve trends comparable to plant values. Zinc was precipitated preferentially through the addition of a lime slurry in BZS precipitation, which was used to maintain the required pH. The main purpose of the effluent treatment step was to remove manganese and magnesium from the process to prevent build-up.
In the solvent extraction step, the zinc was loaded onto D2EHPA according to the following reaction:
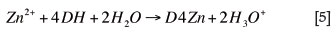
The washing step removed entrained impurities and the above reaction was reversed in the stripping step of solvent extraction. An overall reaction combining the zinc halfreactions was used in electrowinning:
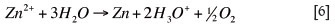
Any stream splits that were unknown were adjusted manually to obtain values comparable to plant data.
Water minimization methodology
Global optimization methods can be divided into two main categories, namely exact methods, which involve numerical, mathematical solutions such as mixed integer non-linear programming (MINLP), and heuristic methods, which use a set of heuristics to arrive at a solution. The heuristics are generally formulated as rules based on experience. Heuristic methods are not always guaranteed to find the best possible solution. However, they are generally more practical than numerical methods, which require excessive amounts of computational time, especially when considering complex process simulations such as the Skorpion Zinc circuit. Although optimization heuristics are limited, process design heuristics are well-established3,4,10 and were used to inform the various optimization scenarios proposed.
The water minimization methodology focused on two main areas, namely unit operational changes and circuit configuration changes. Changes to the unit operations involved improving efficiencies. In practice this could be achieved through the use of better technologies (e.g. high rate thickeners) or through a combination of unit operations (e.g. an extra filtration step) in order to improve the overall efficiency of a process stage4 . The circuit configuration changes were formulated to improve the retention of water in the process, such as increasing the internal recycles to reduce the amount of water lost. This included the addition of extra recycle streams to re-use some of the waste water produced5 . This would subsequently lead to a reduction in fresh water consumed.
Results and discussion
Process simulation validation
Figure 2 is a parity chart of simulated versus plant values for process flows and concentrations. Validation of the process simulation was necessary before investigating the various minimization scenarios. Plant data was available for most of the flow rates but for only the concentrations of major feed and product streams. Concentration data is for zinc and other major metal species. Units are not shown for reasons of confidentiality. However, parity charts compare actual with simulated data and the choice of units is unimportant as these correspond. Note that a log scale is used on both axes in order to compare data over a large range of values on a single figure.
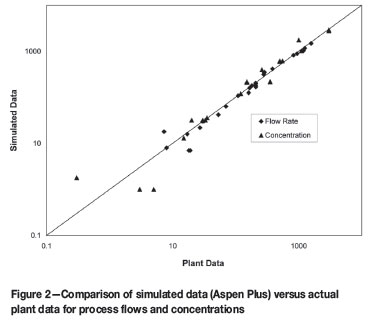
Figure 2 shows that there is generally good agreement between plant and simulated values. Only three of the 52 data points on the figure are far removed from the parity line. These correspond to low concentrations where differences of the order of 1 g/ℓ between plant and simulated data are exacerbated when shown on a log scale. Overall, plant versus simulated data falls within a 10-15% error range, which is not ideal but is considered acceptable for the simulation of such a complex process.
Unit operation changes
Figures 3 to 5 show scenarios which investigate the effect of improving unit operation efficiencies on water consumption. The key output variables investigated were i) zinc production rate ii) water lost to effluent and iii) water lost to filter cake. Stream flow rates have been divided by standard operating flow rates for comparative purposes i.e. to show relative increases/decreases. Here, 100% refers to the standard operating flow rate while values above or below this refer to increases or decreases respectively. So for example, a value of 60% implies that the flow rate of the relevant stream has been decreased to 60% of its standard operating value. Figure 3 shows a scenario where all water inputs to the process were proportionately reduced. As would be expected, this results in a steady reduction in water in both the effluent and filter cake. Water exiting via the effluent shows a more dramatic reduction, since the flow rate of this stream is small compared to that of the cake. The actual amount reduced is comparable to that observed in the cake. However, reducing process water also results in a build-up of salts in the circuit, which affects zinc production. It was found that with more than a 5% reduction in process water, the impurities in the circuit exceed the threshold concentrations. Specifically, a 5% reduction in water resulted in a 10% increase in key concentrations, namely Mg2+, Mn2+ and Cl-. A small reduction in water therefore results in a large increase in contaminants, indicating that this aspect of the Skorpion process is already highly optimized.
Figure 4 shows a scenario where the underflow solids content of the main thickener in the process was increased This results in a decrease in water to filtration, which intuitively should reduce water usage. However, the graph shows that water consumption is largely unaffected and that there is a small decrease in zinc production. The reason for this is apparent when the circuit is analysed systemically. An increase in the solids content of the thickener underflow means that less solution proceeds to BZS precipitation. This in turn results in a smaller recycle of solution to the leaching step, which results in less solution entering thickening. This causes less solution to enter solvent extraction when compared with the base case, which results in slightly solution when compared to the base case. This means that in the BZS filter, which operates on a percentage solids basis, less solution proceeds to effluent. Due to this decrease, a larger proportion of solution is sent back to the main filters to maintain the wash ratio, resulting in a slightly raised water loss to the filter cake. The changes observed from this scenario are therefore balanced by these two effects and the net effect on the process is minimal.
Figure 5 shows a scenario where the efficiency of the main filtration step in the process was increased. This results in a decrease in water to residue in the filter cake, which intuitively should also reduce water usage. Note that due to the nature of the ore, it is not possible to achieve a cake solids content of higher than 70%. The graph shows that, as expected, water to residue in the filter cake decreases, while zinc production remains largely unaffected. However, the decrease in water in the filter cake is offset by an equivalent increase in water in the effluent stream. As indicated previously, the effluent stream shows a more dramatic trend, since the flow rate is smaller than that in the cake. The net effect of this scenario is once again minimal. The unit operational changes explored in Figures 4 and 5 therefore have very little effect on the overall process and merely result in a shift in the circuit water balance.
Circuit configuration changes
Figure 6 shows a scenario where the entire effluent stream is recycled to solvent extraction. Due to the high purity requirement of the water for solvent extraction, the effluent water would have to undergo treatment and demineralization prior to re-use. This scenario should decrease the amount of fresh water required for demineralization. Figure 6 shows that a full recycle results in an increase in water lost via the filter cake, but this effect is negated by the a significant reduction in total fresh water required for solvent extraction. Overall, this scenario results in a 14% reduction in water usage, with no detrimental effect on zinc production.
Figure 7 shows a scenario where the effluent stream is used to supplement pump gland seal water in the circuit. Recycling of effluent results in a build-up of impurities in the circuit, requiring treatment of the effluent stream. The figure shows the reduction in process water that can be achieved circuit, requiring treatment of the effluent stream. The figure shows the reduction in process water that can be achieved with the extent/amount of effluent stream treated, such that impurities in the circuit do not exceed threshold concentrations (Mn2+, Mg2+, Cl-). The graph shows that no untreated effluent can be sent back into the process. However, for a full (100%) treatment of effluent water a 19% reduction in water usage can be achieved before the threshold concentrations are reached. This suggests that Skorpion is currently operating at the limits of water usage, and only through treatment and re-use of waste water can the process be improved. Skorpion has recently begun treating the effluent water through reverse osmosis for re-use in the circuit.
Conclusions
This paper presented a systemic optimization of the water balance of the Skorpion Zinc refinery with the aim of reducing water consumption. From this study the following conclusions may be drawn:
Reducing process water and/or recycling of untreated water is prohibited by the build-up of trace elements which affect product purity
Improving efficiencies in solid-liquid separation operations has a negligible effect on water usage and merely results in a shift in the water balance
Supplementation of demineralized fresh water with demineralized effluent water results in a 14% reduction in water usage
Supplementation of pump gland seal water with fully treated effluent water results in a 19% reduction in water usage.
Skorpion Zinc is operating at the limits of water usage, and can reduce water consumption further only through the treatment and re-use of effluent water. Skorpion has recently begun treating the effluent water through reverse osmosis for re-use.
Acknowledgements
The authors would like to thank both Skorpion Zinc and the Minerals to Metal Research Initiative at the University of Cape Town for supporting this research.
References
1. MUSADAIDZWA, J.M. and TSHININGAYAMWE, E.I. Skorpion Zinc solvent extraction: the upset conditions. Journal of The Southern African Institute of Mining and Metallurgy, vol. 109, 2009, pp. 691-695. [ Links ]
2. FULS, H., SOLE, K., and BACHMANN, T. Solvent extraction in zinc production from a primary source: the Skorpion Zinc experience. Proceedings of ISEC 2005. Academic Journal Electronic Publishing House, 2005. Beijing, China. [ Links ]
3. VAN BERKEL, R. Eco-efficiency in the Australian minerals processing sector. Journal of Cleaner Production, vol. 15, 2007, pp. 772-781. [ Links ]
4. ALVA-ARGAEZ, A., KOKOSSIS, A., and SMITH, R. Wastewater minimisation of industrial systems using an integrated approach. Computers and Chemical Engineering, vol. 22, 1998, pp. 741-744. [ Links ]
5. NORGATE, T. AND LOVEL, R. Water use in metal production: a life cycle perspective. CSIRO Minerals Report, 2004, pp. 1-44. [ Links ]
6. SUDHOLTER, S., REUTER, M., and KRUGER, J. Eco-techno-economic synthesis of process routes for the production of zinc using combinatorial optimization. Metallurgical and Materials Transitions, vol. 27B, 1996, pp. 1031-1044. [ Links ]
8. CIMINI, S., PRISCIANDARO, M., and BARBA, D. Simulation of a waste incineration process with flue-gas cleaning and heat recovery sections using Aspen Plus. Waste Management, vol. 25, 2005, pp. 171-175. [ Links ]
9. SADRAMELI, S. and GOSWAMI, D. Optimum operating conditions for a combined power and cooling thermodynamic cycle. Applied Energy, vol. 84, 2007, pp. 254-265. [ Links ]
10. ZHENG, L. and FURIMSKY, E. ASPEN simulation of cogeneration plants. Energy Conversion and Management, vol. 44, 2003, pp. 1845-1851. [ Links ]
11. MARIANO-ROMERO, C., ALCOCER-YAMANAKA, V., and MORALES, E. Multi-objective optimization of water-using systems. European Journal of Operational Research, vol. 181, 2007, pp. 1691-1707. [ Links ]
Paper received Apr. 2010; revised paper received Mar. 2011.
© The Southern African Institute of Mining and Metallurgy, 2011. SA ISSN 0038-223X/3.00 + 0.00.














