Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.111 n.6 Johannesburg Jun. 2011
TRANSACTION PAPERS
Review of the development work on the Mintek Thermal Magnesium Process (MTMP)
M. Abdellatif
Mintek, Johannesburg, South Africa
SYNOPSIS
The Mintek Thermal Magnesium Process (MTMP) process is based on DC open arc metallothermic smelting of calcined dolomite at normal pressure and temperatures of 1650-1750ºC. The process was demonstrated at the pilot scale in the period 2000-2004. Major changes to the MTMP pilot plant were made throughout the development period, which lasted for about five years. The changes included design and installation of new equipment, modification of existing equipment, changes in the metallurgical approach in terms of feed rates, feed recipes, furnace operating temperatures, cold and hot dolime feeding, and steady and systematic changes in the operating procedures and maintenance of the plant.
As a result, the performance of the pilot plant gradually improved from one campaign to the next, in terms of availability and in particular the condensation efficiency. In Run 10, the process was successfully demonstrated over an 8-day campaign.
Keywords: Magnesium, condensation efficiency, DC arc furnace, silicothermic reduction.
Introduction
Pilot plant testing of the Mintek Thermal Magnesium Process (MTMP) was carried out in the period 2001-2004. The work consisted of 10 campaigns (Runs), each with its own objectives and plans. Between campaigns, certain equipment changes and modifications, and installations of new equipment, were implemented. These changed were guided by the experience gained in earlier campaigns and by theoretical modelling around the new equipment, particularly the condenser. The modelling incorporated momentum, energy, and mass transfer phenomena, including metal vapour condensation in the presence and absence of inert gas (argon). Thermodynamic simulations were also employed for feed recipe manipulation and changes, and for theoretical energy balance calculations around the furnace and the condenser.
In each run, progress was made in terms of improved operability of the pilot plant, and therefore improved metallurgical results, particularly with regard to the condensation efficiency of the volatilized magnesium. This paper highlights the major changes undertaken throughout the testwork that led to the successful demonstration of the process at the pilot scale in 2004. In addition, the major operational and metallurgical results of each run are summarized and briefly discussed.
Background to the MTMP
The MTMP is an advance on the silicothermic technology for magnesium production from calcined dolomite (dolime). In the Mintek process, a DC open arc furnace is employed and the reaction is carried out at atmospheric pressure and at 1700-1750ºC. The MTMP can be carried out in a much more continuous mode than the Magnetherm process, as the volatilized magnesium is recovered as liquid metal in a separate condenser vessel. Slag and some residual ferrosilicon are periodically tapped from the furnace, and magnesium is tapped from the condenser, also intermittently.
The MTMP was initially proven at the 40-80 kW scale in the 1980s where magnesium extraction and condensation at atmospheric pressure were demonstrated1-2. The project was revived in 1999 when Anglo American Corporation (AAC), Eskom, and Mintek decided to pursue the concept at the pilot scale. During the scoping study, it was decided to use the Magnetherm condenser as the basis for the design of the pilot condenser as it was a proven technology, in spite of the fact that Magnetherm process operated on cyclic basis of 10-12 hours each. Therefore, the original objective of the MTMP plant was to extend the cycle time to 20 hours of 'feed on' time. This was later increased to 36 hours for Runs 6-8, and finally to 48 hours for Runs 9-10. The scoping study also concluded that to successfully complete the development work could require three years. In reality, the project lasted for about five years as a result, in part, of extensive and major changes in the design of the pilot plant, particularly the condenser and its peripheral equipment. These changes were considered necessary in order to be able to operate the MTMP plant on a more continuous basis, as compared to the Magnetherm process.
Pilot plant description
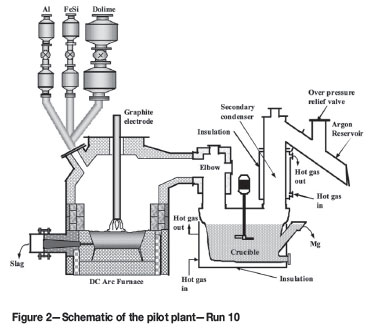
The pilot plant was described in earlier publications3-8. It consisted of a feed system, a 1.5 MW DC power supply, a 1.2 m ID furnace, a condenser with a heating-cooling lead circuit, and an off-gas handling system. In Runs 1-9, the condenser design was based mostly on the Magnetherm process as it was a proven technology (Figure 1). Figure 2 shows the pilot plant as employed in Run 10.
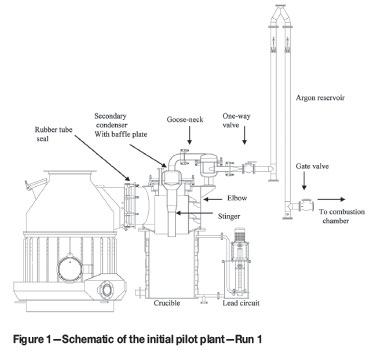
Throughout the testwork, the feed system and the furnace performed very well and therefore no major changes were made. The condenser and the off-gas system, on the other hand, were progressively modified, as a means of increasing the plant 'feed on' availability, and thus to improve on the operational and metallurgical results of the previous runs, as will be shown later.
Testwork
In Runs 1-3, the overall objective was to process 500-600 kg of magnesium-producing recipe (Table I), and thus to give the staff involved sufficient opportunity to become familiar with the plant and the operating procedures, and more importantly to gain experience in magnesium production and its safe handling.
In the first two runs, the dolime was charged into the furnace as received. In Run 3, however, it was preheated to 750ºC in an electrically heated kiln in order to remove any surface moisture and residual carbon dioxide, and then stored in sealed drums under argon atmosphere (as well as in Runs 4-6). Run 3 also excluded the magnesia from the feed. The feed recipe and feed rate were aimed at producing about 80 kg Mg(v) per hour. The original lead circuit was relied on to control the condenser temperatures in these three runs (as well as in Runs 4-5). However, attaining the target temperature of 600-650ºC on the crucible sidewalls was difficult.
As shown in Table I, very little condensation took place inside the condenser crucible. This was attributed to various factors, including oxygen lancing, air ingress, short feeding periods, and the relatively cold condenser due to difficulties encountered with the lead circuit. The condensation efficiency was defined as: {Mg in crude metal/Mg extracted}*100%, where Mg extracted was determined from: {(Mg in feed-Mg in slag)/Mg in feed}*100%.
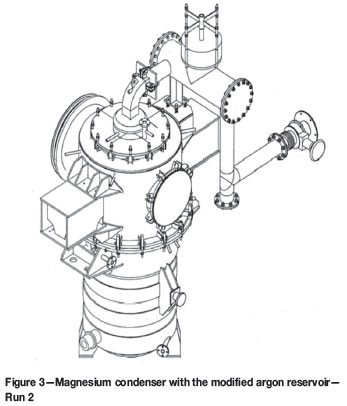
These runs were abandoned as a result of pressure buildup in the facility caused by blockages (mostly Mg/MgO powder) in the off-gas system. In the first run, the blockage was in the U-tube (argon reservoir) and the hot filter. Therefore, prior to Run 2, they were replaced by larger ducting connecting the gooseneck to the combustion chamber (Figure 3). In Run 2, the blockage was mainly in the horizontal section of the argon reservoir, although build-up of solids occurred to some extent in the secondary condenser and the gooseneck areas. Similar experiences were encountered in Run 3.
Runs 4 and 5 aimed at producing 200-300 kg of crude magnesium. This called for feeding about 2 tons of raw materials (Table II). In preparation for Run 4, the crosssectional area of the gooseneck was increased from 100cm2 to 325 cm2 in order to prevent premature blockages in this section. About 2 tons (two batches) of Mg recipe was smelted over a 5-hour feeding period, producing a magnesium ingot weighing almost 200 kg. Attempts to recover the ingot from the crucible were not successful and led to a magnesium fire in the crucible. The crucible contents were therefore reacted with 10% NaOH solution and then with sulphuric acid as a means of oxidizing the metallic magnesium present. Nevertheless, a condensation efficiency of more than 50% was reached in this run, and magnesium extraction was between 82-87%.
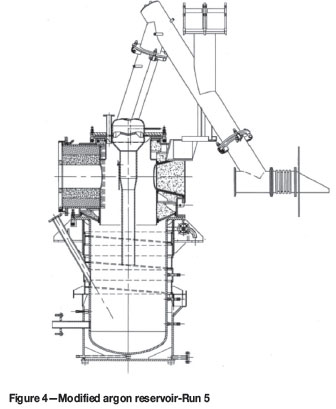
Prior to Run 5, a comprehensive review of the pilot plant and the operating procedures was undertaken. As a result, a dedicated cleaning station was built in order to safely passivate reactive products that formed inside the condenser and the off-gas system. In addition, a modified argon reservoir and gooseneck were designed and installed (Figure 4). The argon reservoir was 200 mm ID, while the gooseneck was replaced with a straight duct (300 mm ID). Prior to the run, the baffle plate inside the secondary condenser was removed.
During the preparation stage, 500 kg of magnesium was melted inside the condenser crucible and then tapped as part of providing training to the staff involved. During the run itself, more than 2.2 tons of raw materials were smelted (Table II) over 5.5 hours of feeding time (2.5 batches). Towards the end of the second batch, a magnesium tap was performed and about 170 kg of crude metal was recovered from the condenser crucible (Figure 5). In addition, about 200 kg of metal was collected when the crucible was treated with molten salt mixture.

This run, as well as Run 4, clearly demonstrated that magnesium condensation at atmospheric pressure was feasible, even in the presence of inert gas (argon with a total flow rate of 40-50 ℓ/min). The calculated condensation efficiency during Run 5 was between 50-55%. Equally significant was the ability to carry out on-line magnesium tapping for the first time.
The relatively low condensation efficiency obtained in Runs 1-5 were related to various factors including: 1) Air ingress and subsequent oxidation of the volatilized magnesium during the operation. This was evident in certain occasions where some of the condenser products contained 0.1-2.0% N2. 2) Low condenser temperatures, particularly at the beginning of the run, resulting in the formation of magnesium powder as well as dendrites. Upon dismantling the condenser for cleaning, such products reacted with air to form oxides and, to a lesser extent, nitrides of magnesium. 3) Oxygen lancing for furnace slag tapping. This led to oxidation of residual magnesium vapour in the system, magnesium powder and dendritic magnesium. 4) Magnesium vapour losses due to back reactions with residual CO2 and moisture in the feed, as well as with CO formed as a result of the reaction between the graphite electrode and the furnace slag.
Thorough evaluation of the metallurgical and operational data highlighted the need for increasing 'feed on' availability and the condensation efficiency over a 'feed on' period of 36 hours and more. Therefore, it was decided to design and install a drill machine and mud gun assembly for furnace tapping (Figure 6), and to remove the slag ladles as quickly as possible by placing them on a sliding plate. Doing so allowed sealing of the furnace taphole and thus faster resumption of feeding into the furnace. In addition, a mechanical plunger was installed on the horizontal section of the argon reservoir as a means of removing solid build-up in this area. For operational and safety considerations, the lead circuit was modified prior to Run 6, whereby the lead reservoir and pump were relocated away from the condenser.

These changes were implemented prior to Run 6, and contributed to the higher condensation efficiency reached in this run (Table III), as well as to increased 'feed on' availability from about 5 hours in the previous run to about 10 hours.
In Run 7, the dolime was heated directly to 700-750ºC in a dedicated heating station that employed two diesel burners and charged hot into the feed system and subsequently into the furnace (Figure 7). The objective was to minimize any residual CO2 (as well as any surface moisture), and therefore to improve the condensation efficiency. For Run 7 and Run 8, the lead jacket around the crucible was replaced by a lead tank as a result of mechanical difficulties. The tank covered the bottom half of the crucible. In addition, propane burners were used to heat the upper sides of the crucible in an attempt to maintain the condenser temperatures at 600-650ºC.

However, the results obtained suggested that the condensation efficiency was lower than that reached in the previous run, although the 'feed on' period was similar. It is believed that during the heating of the dolime, carbon deposition took place, particularly in the colder regions of the vessel. This carbon reacted with the MgO in the slag, generating carbon monoxide, which back reacted with Mg(v), resulting in magnesium losses, and more importantly with the deposition of carbon in the condenser. This led to high levels of magnesium losses in the form of small metallic globules (oxides and salt fluxes) that were trapped within the sludge. Inclusion of aluminium fluoride in the salt flux did not improve the metal recovery from the dross. Therefore, the reported condenser efficiency was lower than that achieved in Run 6.
In order to avoid carbon deposition and to ensure more uniform heating of the dolime, an electrically operated kiln was employed to raise the dolime temperature to 650-750ºC. The dolime was then transferred to the feed system and charged hot into the furnace (typically at 500-650ºC). Doing so resulted in a condensation efficiency of up to 75% in Run 8 (Table III), which lasted for about 10 hours of 'feed on' time. During the run, a few on-line magnesium taps were carried, ranging from 60-110 kg Mg each. In this run, the aluminium addition into the furnace was increased to about 5.5% of the total feed in order to produce a more fluid slag containing 12-13% Al2 O3 for easier and faster furnace tapping.
In spite of the various operational, metallurgical, and mechanical improvements in the previous three runs, blockages in the system persisted and limited the 'feed on' time to only about 10 hours. The blockages occurred mostly downstream of the condenser, and build-up of solids was also evident in the furnace off-gas duct. In addition, the condenser crucible tended to contain a mixture of dross (mostly MgO) and metallic globules, making it difficult to tap the condensed magnesium promptly.
These findings highlighted the need for redesigning the argon reservoir (Figure 8) and the installation of two mechanical plungers on the furnace off-gas and the argon reservoir respectively prior to Run 9. The total feed rate to the furnace was increased by about 25% (Table IV) in an attempt to generate about 100 kg Mg(v)/h. The higher magnesium vapour production rate was intended to provide more energy of condensation into the condenser crucible, and thus to enable tapping of the liquid metal. The dolime was heated in the rotary kiln as in the previous run.
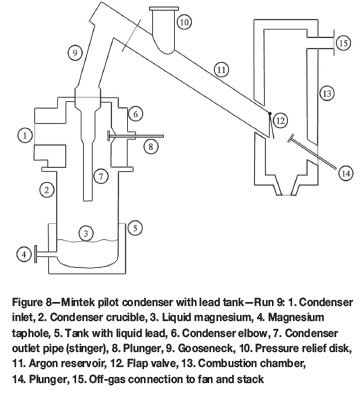
The changes contributed to doubling the feeding period (from 10 hours in Runs 6-8, to about 20 hours in Run 9). During the run, five on-line magnesium taps were successfully performed, yielding more than 500 kg of crude metal. Co-melting of the crucible contents with salt mixture led to the recovery of about 400 kg of magnesium. The calculated magnesium condensation efficiency ranged from 75-80%, which was the highest value obtained thus far. In addition to the improved efficiency, the crude metal was properly assessed with good confidence for the first time, particularly with respect to detrimental impurity elements such as Ca, Si, Fe, and Mn. The levels of such elements in the produced metal were generally lower than those of the Magnetherm crude magnesium9.
However, build-up of solids continued to be a major challenge, particularly in the connection between the argon reservoir and gooseneck area (which led to a major blockage) and in the furnace off-gas duct. Failure of the rubber tube seal towards the end of the run contributed to such build-up. These difficulties limited the run duration to 20 hours, as compared to the target of 48 hours.
Another outcome of Run 9 (as well as Run 8) was the difficulty of keeping the condenser product molten for prompt tapping of the metal. This was related to two major factors: the relatively low condenser temperatures as a result of ineffective and non-uniform heating of the crucible, and to the formation of alternating layers of magnesium and magnesium oxide (Figure 9). In Run 9, lead circulation was not used. Instead, the lead tank (filled with molten lead) was heated using a 250 kW propane burner. The burner was installed underneath the crucible.
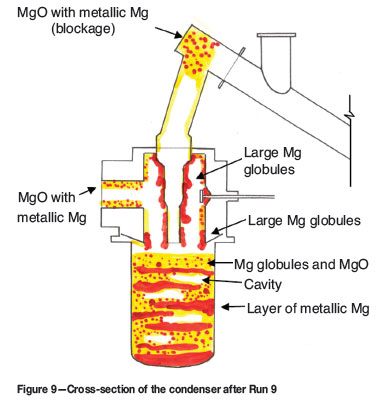
During feeding, and Mg(v) generation, magnesium condensed mostly to the liquid state forming a molten layer. Some of the volatilized metal condensed as powder in colder regions of the condenser. Upon tapping the furnace slag and metal (feed off), the powdery magnesium oxidized, as well as the residual Mg(v) generated during furnace tapping. Oxygen lancing, which was occasionally required to break through the last layer of the frozen slag in the taphole, contributed to the severity of MgO formation. The MgO thus formed settled out from the gas phase on top of the already present metal layer. When feeding commenced, a Mg layer accumulated on top of the MgO layer, and so on. The MgO layer hindered the settling of the liquid metal in the bottom of the crucible, and thus complicated its removal. It should be noted that any solids present in the crucible made it more difficult to heat up the crucible quickly enough. In addition, upon tapping, only liquid metal was removed from the crucible leaving behind most, if not all, of the solids. Eventually, the crucible was filled up with Mg/MgO mixture which could not be removed on-line, causing the termination of the run, in addition to the blockage in the argon-reservoir and the resulting pressure build-up (similar observations were made in Runs 6-8).
Therefore, it was concluded that a radical change to the condenser design was required before any future testwork was to be undertaken. The requirements of such condenser were set as follows: 1) The condenser temperature to be uniform and controllable within the range of 680-750ºC. This was in order to ensure that the condensed metal remained molten all the time and that no powdery or dendritic metal was formed. 2) Any solids arriving at the condenser were required to be in suspension so as they could be removed from the condenser upon metal tapping. Continuous and effective agitation of the magnesium bath was considered to be critical in order to prevent such solids from settling out to form a separate layer at the bottom of the crucible, or from accumulating on top of the magnesium bath to any significant degree. 3) Build-up of solids that could lead to increase pressure and blockages were to be dealt with on-line with the use of mechanical cleaning devices that were operated routinely and/or when a build-up was suspected.
These considerations led to the design of a novel condenser (Figure 2). It consisted of a hot gas inlet section (elbow), a crucible, an off-gas outlet (or secondary condenser), and a dedicated heating system. The heating system consisted of a 250 kW diesel burner, temperature controller, insulated ducting that directed the hot combustion gas in a swirling motion through a gap between the crucible, and an insulating jacket3 .
The condenser assembly was hot-commissioned by melting about 1.2 tons of magnesium ingots and adding a known amount of milled dolime. After agitating the batch for 30 minutes, a sample was taken and analysed. Three batches of dolime totalling about 120 kg, were charged into the crucible. Chemical analysis of the samples taken showed that the liquid magnesium contained between 6-8% solids by mass, suggesting that the stirring action was effective enough to keep the solids in suspension.
These results warranted undertaking the next magnesium production campaign (Run 10). The run was aimed to last for 48 hours of 'feed on' time, and involved charging hot dolime, ferrosilicon, and aluminium (Table IV) for two hours followed by furnace tapping (at a target slag tapping temperature of 1650-1700ºC). These conditions aimed at generating about 100 kg Mg(v)/h (similar to Run 9). During the campaign, about 28 tons of raw materials were smelted over a 52 hour feeding period.
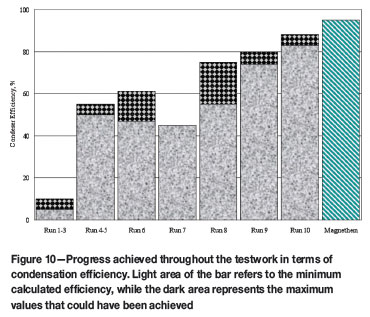
Magnesium condensation was continuously monitored by inspecting the metal level in the crucible spout. Once a sufficient amount had been condensed, the metal was tapped and cast into a 250 ℓ ladle. On average, about 230 kg of magnesium tap was performed after feeding for nearly 3 hours. During the run, 15 on-line magnesium taps were carried out, producing over 3.5 tons of crude metal. In addition, the condensation efficiency achieved averaged about 85% and could have reached at least 88%, in spite of the fact that the argon flow rate into the facility was 70-80 ℓ/min. Given the scale of operation, the condensation efficiency achieved in Run 10 is not significantly lower than that of the Magnetherm process (Figure 10). The reported value for the Magnetherm operation is reached during good operating conditions.
Conclusion
Magnesium extraction in the furnace was high in most of the campaigns, particularly in the last five runs. It reached a level of about 87% as determined based on the slag masses and analysis. However, the actual extraction is believed to have been higher than the stated value, as refractory erosion could not be quantified with reasonable accuracy (the upper section of the furnace sidewalls was lined with MgO-based bricks). In addition, warm-up of the furnace involved charging a slag recipe that contained between 18 and 22% MgO. A certain proportion of this slag always remained in the furnace prior to commencing with the Mg-producing recipe. The level of magnesium extraction did not appear to have been greatly influenced by the high magnesium partial pressure (about 95% of total pressure)in the furnace. In addition to bath stirring induced by the DC arc and the high temperatures characteristics of the arc attachment zone, the relatively high temperature in the furnace (1650-1750ºC) may have driven the reduction reactions to near completion:
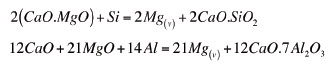
These reactions occur at the bath surface, except when the density of the partially reacted FeSi particle becomes higher than that of the slag, where it continues to react with the MgO of the slag as it perculates through the slag layer1.
The improved condensation efficiency, particularly in Run 10, was attributed to several factors. These include high magnesium vapour partial pressure, bath agitation that improved the contact between the gas phase and the condensing surface (condenser walls and the magnesium bath surface), uniform and steady condenser temperatures, improved sealing of the facility that kept air ingress to a minimum, and steady operation of the plant for a relatively long period of time (8). As metal condensation is controlled by energy transfer, bath agitation contributed to the improved condenser efficiency by quickly dissipating the energy of condensation and thus maintaining a uniform temperature throughout the condenser crucible, particularly the condensing surface temperatures.
The MTMP process is believed to be technologically superior to the Pidgeon plants and may compete well in the Western world with the Chinese producers in terms of the operating costs (8). Savings in operating costs might be possible if a cheaper reductant can be found, such as FeAlSi instead of ferrosilicon. The capital costs of the MTMP plant are higher than the Pidgeon process. Ways to reduce the capital costs of the MTMP plant, particularly the calcining step, are under consideration.
The work summarized in this paper highlights the difficult and challenging path of developing a new technology at the pilot plant level, where detailed engineering solutions are required rather than just a 'proof-of-concept', which is all that can be obtained at the bench scale. The work also indicates a methodology of a process of elimination that is slow and expensive, but is necessary to solve complex problems.
Recommendations
Mintek believes that the operational and metallurgical data collected throughout the testwork can be utilized to design and build a commercial facility of rated at 15 MW, or 15 kt Mg per annum. For larger capacity (>20 MW), a demonstration plant is recommended, although it is not an absolute necessity.
Acknowledgements
The author would like to acknowledge the contribution of the entire staff of Mintek's Pyrometallurgy Division, the AAC team, and Eskom personnel throughout the project execution. The project was funded by the Department of Science and Technology, AAC, Eskom, and Mintek.
References
1. SCHOUKENS, A.F.S. A plasma-arc process for the production of magnesium. Extraction Metallurgy 89, Proceedings of a Symposium organized by the Institute of Mining and Metallurgy, London July, 1989, pp. 209-223. [ Links ]
2. BARCZA, N.A. and SCHOUKENS, A.F.S. Thermal production of magnesium. Assignee: Mintek. United States Patent 4,699,653. October 1987. 5 pp. [ Links ]
3. ABDELLATIF, M.A. Pilot plant demonstration of the Mintek Thermal Magnesium Process. Proceedings of the International Symposium on Magnesium Technology in the Global Age. Pekguleryuz, M.O. and Mackenzie, L.W.F. (eds.), Conference of the Metallurgist, Montreal, Quebec, Canada, 1-4 October, 2006, Metallurgical Society of CIMM, October 2006, pp. 67-80. [ Links ]
4. BARCZA, N.A., FREEMAN, M.J., and SCHOUKENS, A.F.S. Thermal magnesium: is it economically viable?, 2nd Annual Australasian Magnesium Conference, Sydney, Australia, March 2000. [ Links ]
5. BARCZA, N.A., FREEMAN, M.J., SCHOUKENS, A.F.S., and ABDELLATIF, M.A. Pre-feasibility study of the Mintek metallothermic process at Batchelor. 3rd Annual Australasian Magnesium Conference, 2001, Sydney Australia, April 2001. [ Links ]
6. ABDELLATIF, M.A. Mintek Thermal Magnesium Process (MTMP) - Theoretical and Operational Aspects. Southern African Pyrometallurgy 2006 International Conference. Jones, R.T. (ed.). The Southern African Institute of Mining and Metallurgy, Johannesburg, South Africa, 5-8 March 2006, pp. 329-341. [ Links ]
7. SCHOUKENS, A.F.S., ABDELLATIF, M.A., and FREEMAN, M.J. Technological breakthrough of the Mintek Thermal Magnesium Process. The Southern African Institute of Mining and Metallurgy Journal, vol. 106, no. 1, 2006, pp. 25-29. [ Links ]
8. ABDELLATIF, M.A. and FREEMAN, M.J. Mintek thermal magnesium process: status and prospects. Advanced Metals Initiative, Conference organized by the Department of Science and Technology, 18-19 October 2008, Gold Reef City, Johannesburg, South Africa. [ Links ]
9. ABDELLATIF, M. Refining testwork on crude magnesium produced in the Mintek Thermal Magnesium Process. Southern African Pyrometallurgy 2006 International Conference. Jones R.T. (ed.). The Southern African Institute of Mining and Metallurgy, Johannesburg, South Africa, 5-8 March 2006, pp. 343-355. [ Links ]
Paper received Mar. 2010; revised paper received Mar. 2011.
© The Southern African Institute of Mining and Metallurgy, 2011. SA ISSN 0038-223X/3.00 + 0.00.














