Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Communication Disorders
versión On-line ISSN 2225-4765
versión impresa ISSN 0379-8046
S. Afr. J. Commun. Disord. vol.71 no.1 Johannesburg 2024
http://dx.doi.org/10.4102/sajcd.v71i1.1010
ORIGINAL RESEARCH
Swallowing and feeding of young children on high-flow oxygen therapy
Ruhee HoosainI; Bhavani PillayI; Shabnam AbdoolaI; Marien A. GrahamII; Esedra KrügerI
IDepartment of Speech-Language, Pathology and Audiology, Faculty of Humanities, University of Pretoria, Pretoria, South Africa
IIDepartment of Science, Mathematics and Technology Education, Faculty of Education, University of Pretoria, Pretoria, South Africa
ABSTRACT
BACKGROUND: Oral feeding practices of young patients on high-flow oxygen (HFO2) have been controversial. Limited literature exists on this topic, but new studies suggest introducing oral feeds
OBJECTIVE: This study aims to describe the changes in swallowing and feeding of a group of young children on HFO2
METHOD: Twelve participants (mean age 34.17 months [s.d. = 3.97]) on HFO2 were assessed clinically at the bedside using the Schedule of Oral Motor Assessment. Assessments were conducted twice to determine the change in characteristics: upon approval from the managing doctor when respiratory stability on HFO2 was achieved and for a second time on the last day of receiving HFO2 (mean 2.6 days apart). Patients received standard in-patient care and speech therapy intervention
RESULTS: Most participants displayed typical oral motor function at initial and final assessments for liquid, puree and semi-solid consistencies. Purees and soft solid consistencies were introduced to most participants (n = 11, 91.7%). Solids and chewables were challenging for all participants during both assessments. Half of the participants displayed gagging and a wet vocal quality with thin liquids at the initial assessment only
CONCLUSION: This small-scale study found that HFO2 should not preclude oral diets, but in this sample, small amounts of oral feeding could be introduced with caution, in an individualised manner, and with a collaborative multidisciplinary approach. Further research is essential
CONTRIBUTION: Partial oral feeding of specific consistencies was possible during the assessment of young paediatric in-patients on HFO2. Monitoring of individual patient characteristics and risk factors by a specialist feeding team is essential
Keywords: swallowing; feeding; high-flow oxygen; oral motor characteristics; SOMA; speech-language therapist; burns.
Introduction
Respiratory distress in children is often treated using non-invasive ventilation such as high-flow oxygen (HFO2) therapy via nasal cannula (Singer & Rattanachaiwong, 2018). The term high-flow oxygen can be used interchangeably with high-flow nasal cannula (HFNC) oxygen therapy: a non-invasive ventilation method where a humidified combination of oxygen and air is delivered through binasal tubes at gas flow rates of more than 1 litre per minute (Charlton et al., 2022). Published literature on the safety of oral feeding in patients on HFO2 is inconsistent (Barnes et al., 2023; Murphy et al., 2018). Non-invasive ventilation in children increases the diameter of the airway (Murphy et al., 2018). Consequently, there is concern that oral feeding in patients on HFO2 may cause swallowing-related respiratory decline (Maitland et al., 2018; Murphy et al., 2018; Singer & Rattanachaiwong, 2018). There is substantial evidence advocating for early enteral nutrition in patients on non-invasive ventilation (Murphy et al., 2018); however, oral feeding during HFO2 is not yet common practice.
A paucity of research with conflicting conclusions regarding feeding on any form of ventilator support has left speech-language therapists (SLTs) and other health professionals without clear evidence to guide feeding decisions for young children on HFO2 (Murphy et al., 2018). The role of SLTs in paediatric critical care settings, where most patients are on ventilator support, is predominantly in the assessment and management of swallowing and feeding (Murphy et al., 2018; Singer & Rattanachaiwong, 2018). The disadvantage of the initiation of oral feeds while on non-invasive ventilation is that it may induce respiratory distress (Capilouto, 2017). In patients with respiratory distress, reduced energy or possible neurological difficulty could result in oropharyngeal dysphagia (OPD) and possible aspiration (Capilouto, 2017).
Oral intake abstention resulting from HFO2 increases the possibility of oral sensorimotor systems being affected, thus impacting later feeding (Capilouto, 2017). Food refusal and fussy eating may stem from feeding and swallowing dysfunction, unpleasant oral experiences while on non-invasive ventilation, or long-term tube feeding resulting in under usage of oral-motor structures (Capilouto, 2017). A recent stance is that HFO2 should not preclude oral diets, but oral intake should be practised with caution when a child is stable on HFO2 (Singer & Rattanachaiwong, 2018).
Respiratory stability on HFO2 is usually achieved after the flow rate is determined and monitored by a senior physician (Maitland et al., 2018). The weight of the child helps to determine the starting flow rate to achieve respiratory stability on HFO2 (10 kg: 2 L/kg/min [Litres/kilogram/minute] for the first 10 kg plus 0.5 L/kg/min for each kg above 10 kg, with a maximum flow rate of 50 L/min) (Maitland et al., 2018). However, there are no known guidelines in South Africa for SLTs to guide feeding decisions when young children are on HFO2.
There are varying perceptions and practices among members of the multidisciplinary team for feeding ventilator-dependent paediatric in-patients (Barnes et al., 2023). Current published data are predominantly originating in high-income countries with access to HFO2 at healthcare facilities (McCall, 2019; Murphy et al., 2018). South Africa is considered an upper-middle-income country, with a high proportion of people with a low socio-economic status (Sochet et al., 2017).
This under-resourced population is faced with risks such as poor living conditions, and children have an increased number of comorbidities, including lower respiratory tract infections, for which HFO2 may be warranted (Sochet et al., 2017). However, demographically, diagnoses may also be attributed to a lower socio-economic status itself, as people often live and work in environments that have a higher risk for pollution, psychosocial stressors, trauma and injury, and vulnerability to disease with poor outcomes (Marutlulle, 2021).
Research methods and design
Aim and research design
The aim of this study was to describe swallowing and feeding characteristics in a group of young in-patient children on HFO2. This exploratory study set out to describe a preliminary profile of swallowing and feeding characteristics in a small group of young children on HFO2. It consisted of a prospective longitudinal study design with repeated measures at two points to track the change in swallowing and feeding characteristics over time.
Setting
The research site was a large tertiary hospital in South Africa. Participants were from paediatric units which cater for paediatric inpatients who require HFO2 i.e., the paediatric high care unit, paediatric burns unit and intensive care unit. Often, patients in these wards are on HFO2 for various diagnoses such as burn and trauma injuries, chronic lung disease, bronchiolitis, lower respiratory tract infections and severe acute malnourishment. These units accommodated parents only throughout the day's visitation times, where parents often assisted with feeding children if they were not on alternative methods of feeding such as oro-gastric or naso-gastric tubes.
Participants
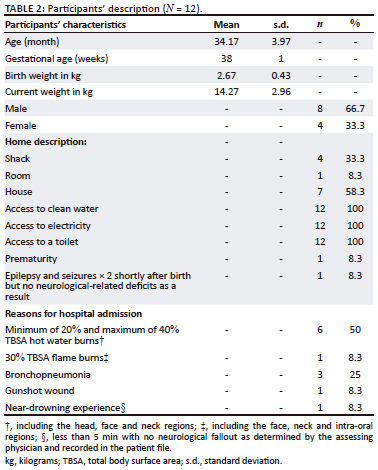
Twelve participants who met the inclusion criteria (Table 1) were prospectively included using snowball sampling (Table 2) as they were referred for assessments from the medical doctor.
Procedure
Data collection took place between June 2021 and December 2021. Participants were seen post-admission after the medical file review, discussion with the managing doctor and when potential participants met inclusion criteria. Participants' caregivers were asked questions in a private space or telephonically. The researcher was adequately qualified and trained in performing the clinical swallowing and feeding assessment and collected data independently. A second rater assessed 15% of the sample. The second rater was a qualified SLT for 4 years, not involved with the study, but trained in the procedures. Inter-rater agreement was obtained at a kappa of 0.981 indicating almost perfect agreement increasing the reliability of the findings.
Each participant was initially assessed at their bedside once respiratory stability was established on HFO2 and once again on the day when participants concluded the HFO2 therapy (i.e. a follow-up assessment, to track changes in swallowing and feeding characteristics). The two assessments were conducted a mean of 2.6 days apart for the participants in this study. The observation of swallowing was ensured to identify visible signs of aspiration such as a maintained drop in the participant's SPO2, coughing, wet vocal quality, intercostal recessions, or difficulty breathing, colour of extremities changing blue as well as a rise in temperature accompanied by sweating (Tosif & Duke, 2017). Verbal feedback was provided to caregivers following assessments. Participants with difficulties were referred to medical and allied healthcare professionals for treatment.
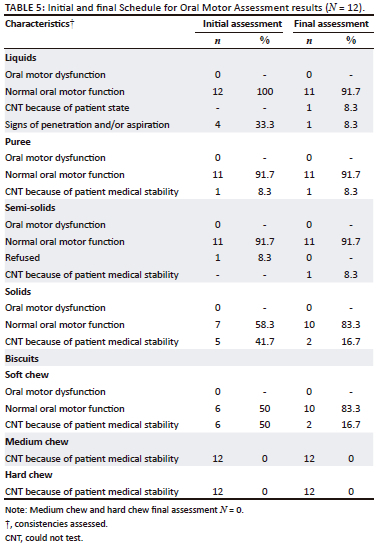
The two assessments were conducted across four food consistencies (liquids, pureed, semi-solid and solid) using the Schedule for Oral Motor Assessment (SOMA) (Reilly et al., 1995). The SOMA was conducted as part of a bedside swallowing and feeding assessment at two points in time, and observations of swallowing were also made. Attempts were made to feed all participants as follows: by cup for liquid consistencies, by spoon for semi-solid and solid consistencies, and independently for orally consuming a finger food (e.g. a biscuit) (Table 3).
Each consistency used in the SOMA was adapted to suit the local context, that is, food that was affordable and known to participants, as in a previous study (Fuls et al., 2020). The International Dysphagia Diet Standardisation Initiative (IDDSI) was used to describe the textures of food and the thickness of liquids used in the assessments of the participants using the SOMA (Cichero et al., 2017). International Dysphagia Diet Standardisation Initiative has established consistency assessments ranging from using a syringe to a spoon or fork in testing a consistency in question to determine where on the continuum of thickness does it lie (scale of 0 [thin consistencies] to 7 [food with no texture restrictions, i.e., hard textures requiring biting and chewing]) (Cichero et al., 2017).
Ethical considerations
Institutional ethical clearance was obtained before data collection commenced at the University of Pretoria, Faculty of Health Sciences, #HUM012/1220.
Results and discussion
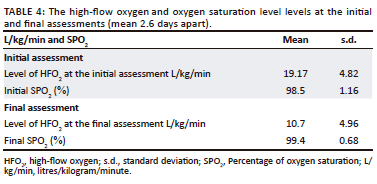
Preliminary information about oral motor function when being introduced to oral feeding on HFO2 was obtained in this small exploratory study of 12 children. Signs of OPD were identified. The two repeated assessments showed changes in participants' ability to be assessed with a wider variety of consistencies on the SOMA. Participants were respiratory stable on HFO2 at a mean level of 19.17 L/kg/min (s.d. = 4.82 L/kg/min) at the initial assessment (Table 4). More than half of the participants managed oral intake of various consistencies at both the initial and final swallowing and feeding assessments within what is expected of their developmental skills at their age (Table 5). Even though participants were medically stable according to inclusion criteria, participants were still displaying behaviours of being weak or lethargic and listless. Therefore, because of the energy required to self-feed and chew the trials of chewable foods, some participants could not be assessed with this owing to visibly presenting poorly or refusing the trial. Subsequently, prior to the final assessment, HFO2 was weaned to a mean level of 10.73 L/kg/min (s.d. = 4.96 L/kg/min) because of participants slowly becoming respiratory stable and coping on lower levels (Table 4). There were two participants who had a decline in their respiratory state requiring them to be re-intubated. These patients could not be assessed because of a decline in their medical stability. These findings are consistent with those of Canning et al. (2021) who concluded that it was not the use of HFO2, but rather patient-specific factors of swallowing and feeding readiness and underlying medical conditions that affected readiness for oral intake.
Previously, there was controversy in feeding children orally on HFO2 and the hesitancy is likely derived from concerns regarding aspiration risk (Murphy et al., 2018). A recent systematic review's findings on oral feeding while on HFO2 were insufficient to provide a concluding stance (Canning et al., 2021). Thus, to date, the effects of HFO2 on swallowing and feeding are still not well elucidated, which results in varied feeding practices and intervention recommendations provided by SLTs (Rice & Lefton-Greif, 2022). This small-scale study found that HFO2 should not preclude oral diets, but in this sample, small amounts of oral feeding could be introduced with caution, in an individualised manner, while collaborating closely with the multidisciplinary team involved. Further research would be beneficial. Instrumental assessment would be of value to determine the presence of silent aspiration.
Prior to the initial assessment, the treating physician provided participants with medical clearance to partake in oral feeding. Participants were found to be awake, alert, and responsive and were repositioned into a semi-reclined position for the assessment. All participants had GCS between 12 and 15 at both assessments. The findings show that those participants who could be assessed (n = 10), displayed mostly normal oral-motor function for liquids, pureed and semi-solid consistencies at both initial and final assessments of swallowing and feeding (Table 5). The volumes of trials offered varied as tolerated by participants. Feeding difficulties that were identified in this sample were: refusal of certain consistencies requiring increased effort to swallow, or the participant's medical state contributing to reduced or no oral intake, and an increased duration of feeding. Liquid consistencies (IDDSI level 0) may have contributed to a possible aspiration event when the amount of high flow oxygen was increased (mean 19.17 L/kg/min; s.d. = 4.82 L/kg/min) as coughing was observed. This would have to be confirmed using a larger sample. While the feasibility of instrumental assessment remains an issue in a country such as South Africa where videofluoroscopic swallow studies are not readily available (Coutts & Pillay, 2021), it will be valuable to conduct future research with similar populations using instrumental assessments to determine the presence of silent aspiration.
In this study, the liquid consistency put participants at a possible risk of aspiration at the initial assessments (n = 4, 33.3%). The liquid consistency was safely administered at the final assessment while still on HFO2. Pureed, semi-solid and solid consistencies were safely administered when respiratory stability was achieved following the initiation of HFO2. This also proved to be the case when HFO2 was being weaned because of slowly achieving respiratory stability in those participants who were medically and physically stable to feed orally. The less viscous the liquid consistency, the higher the chances of an aspiration event without oxygen therapy (Wolter et al., 2018). Therefore, with oxygen therapy and a higher flow rate, thin liquids may be more difficult to control while swallowing (Steele et al., 2015).
Purees and semi-solids were tolerated well in all participants at both assessments. Liquids and soft chew consistencies were only possible at the second attempt. The SLTs could therefore, consider a careful approach to liquid trials with young patients on HFO2 in future research endeavours. Evidence suggests that thicker consistency trials such as IDDSI level 2 prior to thin liquid trials may be a consideration to reduce possible penetration and/or aspiration events, however, post-swallow pharyngeal residue should ideally be monitored using instrumental means (Steele et al., 2015), which is not always an option at hospitals in South Africa. In the study, participants who orally consumed thicker consistencies and solids (IDDSI levels 2-6) appeared to have swallowed successfully and did not display observable signs of oral residue post-swallow. Solids and chewable consistencies (IDDSI level 7) were not introduced successfully in this study, often because of participants' medical state.
While this study's findings are based on a small sample, the value of the findings is that it paves the way for future research. A two-point measurement showed change in the swallowing and feeding ability of participants. Generalisability of findings will not be possible, but a preliminary oral feeding framework was conceptualised based on the observations from this small-scale local study (Figure 1). Participants may have coped on smaller amounts of soft food because of their medical stability and overall state. Clinically, this could be suggestive of 'partial oral feeds' on HFO2, that is, orally feeding consistencies that the individual coped on at the assessment supplemented by enteral feeds as a means of maximum nutritional intake (Wolter et al., 2018). In this study, participants made progress from the first assessment to the second assessment evidenced by the number of participants who were able to take liquid and soft chew consistencies at the second attempt. Oral feeding ability should thus be specified for the consistency and the amount used. Close consultation with a medical doctor and dietician is necessary. Larger prospective studies are also warranted in this regard. It is important that the multidisciplinary feeding team considers patients holistically and identifies oral feeding as a common goal while working together to ensure nutrition is provided safely (Rice & Lefton-Greif, 2022).
The prominent environmental risk in this study was being born into families with a low socio-economic status from informal settlements. Most participants in this study had trauma-related burns to the body (maximum TBSA of 40%) because of hot water burns. Furthermore, a history of bronchopneumonia proved to be another frequent reason for hospital admission in this study. Such diagnoses may be attributed to a lower socio-economic status as people often live and work in environments that have higher daily living risk factors (Marutlulle, 2021). The observations in this study showed participants on HFO2 who commenced oral trials successfully were weaned from HFO2 quicker than participants who did not, but well-designed comparative studies are necessary to confirm this finding. The commencement of oral feeding while on HFO2 is slowly being supported as it could facilitate transition to full oral feeding without adverse events (Singer & Rattanachaiwong, 2018). Findings in the current exploratory study are insufficient to conclude this stance. The value of the findings are the age of participants studied (mean = 34.17 months) for which there is limited published data, although the sample was small. Larger prospective studies will be valuable.
Considering the role of SLTs within the paediatric intensive care unit, where professionals are faced with assessments for oral feeding readiness with young children on HFO2 as the middle ground between either intubation or nasal prongs or room air. The study provides preliminary findings on which further research may be based. SLTs may attempt oral feeding assessment using the puree, semi-solid or solid with young children cautiously, but more confidently than before. However, careful monitoring and individualised decisions remain important. Findings are useful, but further research is required to support SLTs in making complex feeding decisions for high-risk children on HFO2.
Studies employing instrumental assessments such as videofluoroscopy or flexible endoscopic evaluation of swallowing could be of value but are not readily available to South African SLTs. SLTs and feeding teams must proceed cautiously, considering the complexity of oral feeding in patients with respiratory distress. Team-based decisions and inter-professional collaboration remain crucial for all healthcare professionals working with swallowing and feeding in vulnerable populations.
Conclusion
This study's findings are not generalisable, but is the starting point for further prospective research on the paediatric population requiring HFO2. Repeated measures in this longitudinal study showed changes in the participants' ability to tolerate liquid and soft chew consistencies. Findings highlighted patient-specific determinants such as the medical diagnosis, reason for admission or state of alertness, often lead to respiratory compromise affecting patients' readiness for different consistencies of oral alimentation. Prospectively collected data were obtained providing valuable information, which is supportive for future research efforts. The findings may be valuable to all healthcare providers such as SLTs working with children on HFO2 within similar settings.
Acknowledgements
The authors would like to acknowledge the patients who were participants in this study from 2020 to 2022, the caregivers of these patients and the site at which the research was conducted. This article is partially based on the author's thesis for the degree of Master's in Speech Therapy and Audiology, at the University of Pretoria, South Africa, with advisor E. Kruger and coadvisors B, Pillay and S. Abdoola available here: https://repository.up.ac.za/handle/2263/88963
Competing interests
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article.
Authors' contributions
This study was conceptualised, prepared and written up by R.H., B.P., S.A. and E.K. except M.A.G. mainly assisted with the statistical analysis of the data obtained from this study. R.H. collected the data, and this was towards her Master's degree in speech-language pathology.
Funding information
The data that support the findings of this study are available upon reasonable request from the corresponding author, R.H.
Data availability
The data are only accessible to the researchers who are the authors of this study.
Disclaimer
The written outcome, opinions and views expressed in this article are those of the authors and do not reflect the official guideline and policy of any affiliated agency of the authors.
References
Barnes, C., Herbert, T.L., & Bonilha, H.S. (2023). Parameters for orally feeding neonates who require noninvasive ventilation: A systematic review. American Journal of Speech-Language Pathology, 32(4), 1-20. https://doi.org/10.1044/2023_ajslp-22-00259 [ Links ]
Canning, A., Clarke, S., Thorning, S., Chauhan, M., & Weir, K.A. (2021). Oral feeding for infants and children receiving nasal continuous positive airway pressure and high flow nasal cannula: A systematic review. BMC Pediatrics, 21(1), 83. https://doi.org/10.1186/s12887-021-02531-4 [ Links ]
Capilouto, G.J. (2017). Pediatric dysphagia. Seminars in Speech and Language, 38(2), 75-76. https://doi.org/10.1055/s-0037-1599104 [ Links ]
Charlton, M.E., Peterson, S.J., LaGorio, L.A., Mirza, S.H., & Scott, J.B. (2022). A survey of feeding practices during high-flow nasal cannula Oxygen Therapy. Respiratory Care, 68(9), 1229-1236. https://doi.org/10.4187/respcare.10469 [ Links ]
Cichero, J.A.Y., Lam, P., Steele, C.M., Hanson, B., Chen, J., Dantas, R.O., Duivestein, J., Kayashita, J., Lecko, C., Murray, J., Pillay, M., Riquelme, L., & Stanschus, S. (2017). Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: The IDDSI framework. Dysphagia, 32(2), 293-314. https://doi.org/10.1007/s00455-016-9758-y [ Links ]
Coutts, K., & Pillay, M. (2021). Decision making and the bedside assessment: The speech language therapists' thinking when making a diagnosis at the bed. South African Journal of Communication Disorders, 68(1), 1-8. https://doi.org/10.4102/sajcd.v68i1.790 [ Links ]
Fuls, N., Krüger, E., & van der Linde, J. (2020). Feeding characteristics of infants in a lower-middle-income country. Journal of Paediatrics and Child Health, 56(7), 1083-1089. https://doi.org/10.1111/jpc.14823 [ Links ]
Jain, S., & Iverson, L.M. (2019). Glasgow Coma Scale. StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK513298/
Maitland, K., Kiguli, S., Opoka, R.O., Olupot-Olupot, P., Engoru, C., Njuguna, P., Bandika, V., Mpoya, A., Bush, A., Williams, T.N., Grieve, R., Sadique, Z., Fraser, J., Harrison, D., & Rowan, K. (2018). Children's Oxygen Administration Strategies Trial (COAST): A randomised controlled trial of high flow versus oxygen versus control in African children with severe pneumonia [version 2; referees: 2 approved]. Wellcome Open Research, 2, 100. https://doi.org/10.12688/wellcomeopenres.12747.2 [ Links ]
Marutlulle, N.K. (2021). A critical analysis of housing inadequacy in South Africa and its ramifications. Africa's Public Service Delivery and Performance Review, 9(1), a372. https://doi.org/10.4102/apsdpr.v9i1.372 [ Links ]
McCall, E. (2019). High flow respiratory support for an infant, child, or young person. Starship Hospital Guidelines.
Murphy, R., Harrison, K., & Harding, C. (2018). Feeding infants on high flow nasal cannula oxygen therapy: Exploration of speech-language pathologists' decision-making processes. Journal of Clinical Practice in Speech-Language Pathology, 20(3), 121-127. Retrieved from http://ezproxy.lib.utexas.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=133598090&site=ehost-live [ Links ]
Reilly, S., Skuse, D., Mathisen, B., & Wolke, D. (1995). The objective rating of oral-motor functions during feeding. Dysphagia, 10(3), 177-191. https://doi.org/10.1007/BF00260975 [ Links ]
Rice, J.L., & Lefton-Greif, M.A. (2022). Treatment of pediatric patients with high-flow nasal cannula and considerations for oral feeding: A review of the literature. Perspectives of the ASHA Special Interest Groups, 7(2), 543-552. https://doi.org/10.1044/2021_PERSP-21-00152 [ Links ]
Sharma, S., Danckers, M., Sanghavi, D., & Chakraborty, R.K. (2021). High flow nasal cannula. Physiological effects and clinical applications. StatPearls Publishing. https://doi.org/10.1007/978-3-030-42454-1
Singer, P., & Rattanachaiwong, S. (2018). To eat or to breathe? The answer is both! Nutritional management during noninvasive ventilation. Critical Care, 22(1), 27. https://doi.org/10.1186/s13054-018-1947-7 [ Links ]
Sochet, A.A., McGee, J.A., & October, T.W. (2017). Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hospital Pediatrics, 7(5), 249-255. https://doi.org/10.1542/hpeds.2016-0131 [ Links ]
Steele, C.M., Alsanei, W.A., Ayanikalath, S., Barbon, C.E.A., Chen, J., Cichero, J.A.Y., Coutts, K., Dantas, R.O., Duivestein, J., Giosa, L., Hanson, B., Lam, P., Lecko, C., Leigh, C., Nagy, A., Namasivayam, A.M., Nascimento, W.V., Odendaal, I., Smith, C.H., & Wang, H. (2015). The influence of food texture and liquid consistency modification on swallowing physiology and function: A systematic review. Dysphagia, 30(2), 272-273. https://doi.org/10.1007/s00455-015-9603-8 [ Links ]
Tosif, S., & Duke, T. (2017). Evidence to support oxygen guidelines for children with emergency signs in developing countries: A systematic review and physiological and mechanistic analysis. Journal of Tropical Pediatrics, 63(5), 402-413. https://doi.org/10.1093/tropej/fmw100 [ Links ]
Wolter, N.E., Hernandez, K., Irace, A.L., Davidson, K., Perez, J.A., Larson, K., & Rahbar, R. (2018). A systematic process for weaning children with aspiration from thickened fluids. JAMA Otolaryngology - Head and Neck Surgery, 144(1), 51-56. https://doi.org/10.1001/jamaoto.2017.1917.s [ Links ]
 Correspondence:
Correspondence:
Ruhee Hoosain
hoosainruhee@gmail.com
Received: 28 Aug. 2023
Accepted: 07 Dec. 2023
Published: 02 Mar. 2024














