Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Journal of Laboratory Medicine
On-line version ISSN 2225-2010
Print version ISSN 2225-2002
Afr. J. Lab. Med. vol.12 n.1 Addis Ababa 2023
http://dx.doi.org/10.4102/ajlm.v12i1.2172
ORIGINAL RESEARCH
Storage of Mycobacterium tuberculosis culture isolates in MicrobankTM beads at a South African laboratory
Anura DavidI; Lesley E. ScottI; Pedro Da SilvaII; Elizabeth MayneII, III; Wendy S. StevensI, II
IWits Diagnostic Innovation Hub, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IINational Priority Program, National Health Laboratory Services, Johannesburg, South Africa
IIIDivision of Immunology, University of Cape Town, Cape Town, South Africa
ABSTRACT
BACKGROUND: Mycobacterium tuberculosis complex (MTBC) isolates are typically stored at −70 °C in cryovials containing 1 mL aliquots of a liquid medium, with or without 50% glycerol. Multiple uses of the culture stock may decrease the strain viability while increasing the risk of culture contamination. Small culture aliquots may be more practical; however, storage capacity remains challenging. MicrobankTM beads (25 beads/vial) for the long-term storage of fungal cultures is well documented, but their use for storing MTBC isolates is uninvestigated
OBJECTIVE: The study aimed to determine the feasibility of using MicrobankTM beads for long-term storage of MTBC isolates at a laboratory in South Africa.
METHODS: In February 2020, 20 isolates in liquid culture were stored in MicrobankTM beads, following an in-house developed protocol, at −70 °C. At defined time points (16 months [15 June 2021] and 21 months [18 November 2021]), two beads were retrieved from each storage vial and assessed for viability and level of contamination.
RESULTS: Stored liquid isolates demonstrated MTBC growth within an average time-to-detection of 18 days following retrieval, even at 21 months post storage. Contaminating organisms were detected in 2 of 80 (2.5%) culture isolates.
CONCLUSION: MicrobankTM beads will allow for the reculture of up to 25 culture isolates using a reduced culture volume compared to current storage methods. MicrobankTM beads represent a storage solution for the medium-term storage of MTBC isolates.
What this study adds: This study evaluated the use of MicrobankTM beads as an alternate method for storing MTBC culture isolates at −70 °C and provided a suitable option for medium-term storage of MTBC.
Keywords: Mycobacterium tuberculosis; culture isolates; storage; liquid culture; Microbank beads.
Introduction
Tuberculosis diagnosis is a challenge in many health centres. The introduction and subsequent endorsement by the World Health Organization of the GenoType MTBDRplus (HAIN Lifesciences, Nehren, Germany),1 Xpert MTB/RIF and Xpert MTB/RIF Ultra assays' (Cepheid, Sunnyvale, California, United States),2,3 and the Truenat MTB, MTB Plus and MTB-RIF Dx assays (Molbio Diagnostics, Goa, India)4 highlighted the advantages of using molecular technologies for diagnosis. Although there is a lag in developing new tuberculosis diagnostic assays, in July 2021, another group of technologies received the World Health Organization's conditional recommendation for use in tuberculosis diagnosis.5 These are cobas MTB and cobas MTB-RIF/INH (Roche, Basel, Switzerland),6 Fluorotype® MTBDR (Bruker, Nehren, Germany),7 RealTime MTB (Abbott, Illinois, United States),8,9 and the BD MAX MDR-TB assay (Becton Dickinson, Franklin, New Jersey, United States).10 The Treatment Action Group report provides information on current and new screening and triage tools.11 The expanding tuberculosis diagnostics pipeline and other non-molecular, culture-based, microscopy and radiology innovations can increase testing and earlier disease detection, even in difficult-to-detect cases, such as in HIV co-infected individuals; expand the spectrum of drug-susceptibility testing; and reduce costs.12 Once a technology becomes available, it must be validated to ensure conformity to the manufacturer's claims and/or target product profiles and verified to assess suitability within the intended settings.13,14,15,16,17,18 Both validation and verification require suitable reference material that is stable and can be safely and efficiently transported. Mycobacterial species pose a potential biosafety risk, especially for staff responsible for storing and transporting or aliquoting isolates. Culture isolates should also be stored in an appropriate medium and at the correct temperature to maintain viability. These requirements have both cost and space implications.
One of the earliest studies on long-term storage and preservation of mycobacteria was performed in 1972.19 Mycobacterium tuberculosis complex (MTBC) (cultured on Proskauer and Beck medium) and Mycobacterium bovis (cultured on Sauton's medium) were stored in nine different diluents. These included sterile skim milk; 1% gelatin buffered at pH 6.8; 1:5 dilution of Sauton's medium; 0.25% Triton WR 1339 in Sauton's medium; a solution containing 8.3% dextran, 7.5% glucose, and 0.025% Triton WVR 1339; 15% aqueous solution of lactose at pH 5.0; 5% sodium glutamate; Tween-albumin medium; and distilled water. Archive stocks were stored at two different temperatures (−20 °C and −70 °C) for 3 years. There was a significant reduction in MTBC isolate viability when stored at −20 °C versus −70 °C. In addition, the methods applied had safety concerns, such as the bottling procedures, appropriate container selection, transportation, and the risk of aerosolisation (once the bottle was opened). Although most of these issues were resolved, the storage media and safety considerations were not ideal.19
In 2005, Huang et al. demonstrated that MTBC isolates from solid media could be stored at −70 °C in 7H9 broth without significant loss in viability (> 90%) for up 7 years, while the viability of strains preserved directly from mycobacteria growth indicator tubes (MGITs) was acceptable for up to 3 years.20 In 2014, the World Health Organization released a laboratory manual recommending MTBC isolate storage in 7H9 broth with glycerol,21 media preparation, specific equipment, and sterile work areas. Although the World Health Organization-recommended technique efficiently stores and maintains MTBC strain viability, Metcalfe et al. and Hanekom et al. reported a possible loss of strain fitness due to the subsequent need for sub-culture to maintain stock culture isolates.22,23 Furthermore, subculturing is prone to contamination, which may further risk the loss of valuable isolates. Kremer et al. reported an alternative option to collect samples from the surface of a frozen medium by scraping the top layer without defrosting the remaining suspension.24 However, the use of a frozen stock may reduce the viability of isolates if the entire tube thaws during the subculturing process. Glass beads have demonstrated medium-term storage for MTBC with strain recovery rates of > 94% within 30 days of incubation by Giampaglia et al.25 However, both the beads and storage vial require pre-sterilisation and preparation of storage medium, which increases the process cost and is labour intensive.
The MicrobankTM platform (www.https://pro-lab.com/products/clinical-microbiology/bacteriology/microbank/) has approximately 25 sterile beads per storage vial and a specifically formulated cryopreservative for low-temperature storage. It is potentially a suitable alternative storage solution for MTBC isolates. The MicrobankTM beads are porous, allowing microorganisms to adhere to the bead surface. A new culture can be grown by inoculating culture media with a single bead from the storage. Thus, the process can be repeated for the 25 beads of each isolate compared to the current method, where the number of possible new cultures depends on how much of the stock is used for each reculture. Some studies26,27,28 have reported reasonable fungal isolate recovery rates (96% - 100%) from the MicrobankTM beads; however, as far as we know, the MicrobankTM storage solution has not been assessed for MTBC isolate recovery. Nevertheless, the MicrobankTM beads may maintain longer MTBC viability and allow maximum usage with 25 beads, reducing process cost and preserving precious culture isolates.
This study aimed to determine the feasibility of using the MicrobankTM beads to store MTBC isolates from a laboratory in South Africa.
Methods
Ethical considerations
Ethics approval to store MTBC isolates from residual MTBC-positive liquid culture on the MicrobankTM system was obtained from the Human Research Ethics Committee at the University of the Witwatersrand in South Africa (ethics approval number: M1511110). The MGIT culture isolates were allocated a study number to maintain participant confidentiality. Informed consent was, therefore, not required. Isolates were described based solely on their laboratory characteristics, including MGIT Ziehl Neelsen, blood agar, line probe assay and drug-susceptibility testing.
Preparation of culture isolates for MicrobankTM bead storage
Twenty residual MTBC-positive culture isolates from the Department of Molecular Medicine and Hematology research laboratory at a university in South Africa, containing a heavy initial inoculum (approximately 105 to 106 colony-forming units per millilitre [CFU/mL]) from MGITs were selected. The time-to-detection (TTD) for each culture isolate was determined and provided by the BACTEC™ MGIT 960 Mycobacteria Culture System (Becton Dickinson, Sparks, Maryland, United States) up to 42 days after incubation. Isolates comprised rifampicin- and isoniazid-susceptible, multidrug-resistant, and rifampicin-only or isoniazid-only resistant strains. An ATCC 25177 strain (Davies Diagnostics, Johannesburg, South Africa) was included as a positive control. Before their storage on the MicrobankTM beads (Pro Lab Diagnostics, Texas, United States) in February 2020, the liquid culture isolates were stored in their original tubes at room temperature for ~5 months following removal from the BACTEC™ MGIT 960 System. The MGITs were vortexed for 30 s to break up clumps before removing 1 mL of liquid culture. The bead inoculum was prepared by centrifuging 1 mL of liquid culture at 3000 g for 15 min at room temperature. A volume of 500 μL of supernatant was removed, and the remaining 500 μL of the re-suspended pellet (using a pipette) was added to one MicrobankTM bead vial. The resulting suspension was then inverted 10 times and incubated at room temperature for 60 min allowing the mycobacteria to adhere to the bead surface. The liquid was then aspirated, and the vial was stored at −70 °C per manufacturer's instructions.29 At defined time points (16 months [15 June 2021] and 21 months [18 November 2021]), two beads were aseptically removed with a pipette tip from each isolate vial. The beads were then added to two separate MGITs containing 0.8 mL of polymyxin B, amphotericin B, nalidixic acid, trimethoprim and azlocillin antibiotic mixture (Becton Dickinson, Sparks, Maryland, United States) and incubated in a BACTEC™ MGIT 960 System at 37 °C for a maximum of 42 days. For those culture isolates that flagged positive, the presence of acid-fast bacilli was confirmed by smear microscopy (Ziehl Neelsen stain).30 The presence of contaminating organisms were confirmed by visual inspection of any 'abnormal' growth in the MGITs (where possible) and/or plating an aliquot of the culture onto blood agar and then analysing the plate for any growth after 48 h of incubation at 37 °C. A MGIT TBc Identification MPT64 antigen test (Becton Dickinson, Sparks, Maryland, United States) test was used to confirm the presence of MTBC in the MGIT isolates. Staff performing the storage and retrieval procedure were asked to compare the MicrobankTM procedure (in terms of ease to use) to the glycerol procedure currently used in the laboratory.
Data analysis
The MGIT TTD for each bead was obtained from the BACTEC™ MGIT 960 printout. The TTDs were captured on a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, Washington, United States). A one-way analysis of variance was performed on the culture isolate TTD using SAS (SAS Institute Inc 2013. SAS/ACCESS® 7.1 Interface to ADABAS, Cary, North Carolina, United States) to determine if there were significant differences at each time point. In addition, the number of archive beads that successfully grew MTBC, the number contaminated, and the number that failed to grow were reported.
Results
After a storage period of 16 and 21 months, 34 of 40 (85%) archive beads successfully grew MTBC, 5 of 40 (12.5%) did not demonstrate growth after the 42-day incubation, and 1 of 40 (2.5%) demonstrated contamination (Figure 1). The baseline TTD for the positive control culture isolate, ATCC 25177, was 15.9 days (Table 1). The positive control demonstrated a mean TTD of 8.4 days and 20.5 days after 16 months and 21 months. For isolates, where both beads retrieved at the same time point, demonstrated growth of MTBC, 15 of 16 and 13 of 15 after 16 and 21 months showed similar TTDs (Figure 1). Isolates 4 and 17 demonstrated > 8-day difference in TTD between the two beads retrieved simultaneously. The box and whisker plot (Figure 1) displays the distribution of means representing the TTD for the original culture, month 16 and month 21. The one-way analysis of variance indicated a significant difference among the means of the original, month 16 and month 21 TTD (F (2, 57) = 9.17, p < 0.001) (Online Supplementary file Table 1). Post-hoc analysis using Tukey's Honestly Significant Difference test revealed that the original TTD (mean = 8.06 days) was significantly lower than both month 16 (mean = 19.18 days) and month 21 (mean = 17.10 days) TTD. However, no significant difference was observed between the mean culture isolate TTD at month 16 and month 21.
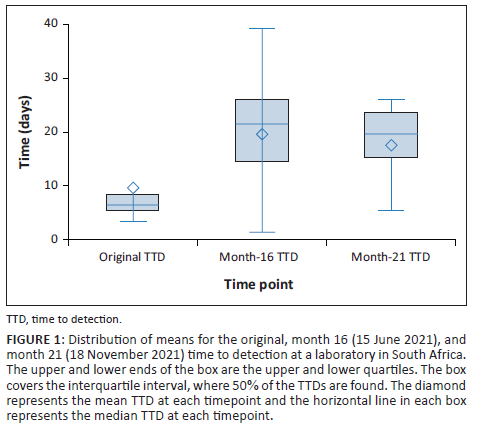
Regarding usability, laboratory staff reported that the MicrobankTM beads were technically undemanding (requiring only routine laboratory procedures such as centrifugation) and easy to use. Furthermore, no additional preparation of reagents was required.
Discussion
Storage of MTBC culture isolates on the MicrobankTM beads was performed at −70 °C as recommended19,21,31 and MTBC isolates were recovered within 39 days of incubation in the BACTEC™ MGIT™ 960 instrument with minimal contamination. For archive cultures where contamination was seen, culture isolates grown from the second bead from the same vial did not show contamination suggesting that contamination occurred during the MGIT reculture process rather than during processing for storage. Since the MicrobankTM bead platform doesn't address the possible contamination problem due to multiple openings and closing of the vials, sterility during the reculture process is critical.
Although the statistical analysis showed a significant difference in the TTD between the original and post-storage TTD, the TTD between the 16-month and 21-month time points was not statistically significant. This increased TTD could be attributed to decreased metabolic activities of the MTBC bacilli due to their storage at low temperatures.31 There was variability in TTD at both time points post storage, even in beads from the same vial, which may suggest that growth depends on the number of bacilli adhering to the bead. Rather than a loss in viability, a decreased number of bacilli on some beads may also be the reason for some culture isolates demonstrating a lack of growth after the 42-day incubation period and highlighting the importance of the uniform distribution of bacteria. It is therefore essential to rigorously invert the MicrobankTM vial at the time of inoculation to assist with the uniform distribution of the bacteria across the beads. The TTD has been described as a time-to-event variable, which represents an indirect measurement of the bacterial load,32 and it is possible to reduce the TTD by increasing the colony-forming units of the initial inoculum. Increasing colony-forming units may be achieved by incubating the culture isolates at 37 °C for a more extended period to allow for increased growth or increasing the starting volume of the inoculum. The susceptibility profile of the culture isolates also did not appear to impact the viability of the culture isolate or the TTD.
Recovery rates of MTBC preserved from culture media (stored at −70 °C for 7 years) can vary from 97.4% to 100%.20 Room temperature storage of culture isolates has also been investigated.33 Although the bacteria remained viable for up to 6 years, recovery rates were low (54.0% to 66.4% depending on storage conditions, such as the availability of an air conditioner) with a contamination rate of ~7%. Recovery rates of MTBC using the microbead method after a 21-month storage period were demonstrated to be 85%. Although this lower recovery rate appears to be a limitation of this method, further investigation is required using an increased inoculum volume or a higher culture concentration and a longer storage period.
A key advantage of the Microbank™ beads is their safety profile. The minimal liquid volume reduces the risk of leakage and subsequent aerosolisation. The system also does not require the preparation and autoclaving of media. Due to the increased risk of defrosting the entire vial and potential loss of the stock culture isolate, current practice recommends storing two vials of stock material in two separate freezers, which already has cost and space implications.34 With the Microbank™ bead storage, 25 individual isolate recoveries can be made from a single vial, allowing efficient storage, which is ideal for space-constrained environments. With routine subcultures, it has been reported that frequent subcultures may distort drug-susceptibility levels and patterns of strains,35 but with the Microbank™ system, since the primary culture isolate is technically not sub-cultured, this risk is minimal. For ease of identification within biorepository settings, MicrobankTM beads are available in a variety of colours.
Although not investigated in this study, MicrobankTM beads provide an attractive alternative for transporting viable isolates and for applications such as proficiency testing. The current transport of infectious MTBC culture isolates for further confirmatory reflex testing, such as sequencing, requires strict safety adherence criteria and is costly. Typically a secondary watertight container with absorbent material, together with outer packaging,36 is employed for shipping isolates and limitations are placed on the total allowable volume of liquid material in a single shipping container.37 The MicrobankTM bead platform will require the same safety considerations as conventional storage but offers a reduced risk of aerosolisation since the beads are not transported with any liquid.
Limitations
This study has some limitations, the most important being the absence of long-term storage data and a small sample size. Investigation into the use of MicrobankTM beads for transporting MTBC was not performed.
Conclusion
The use of MicrobankTM beads for medium-term storage of MTBC isolates at −70 °C shows promise, with growth observed within ~5 weeks of resuscitation, even at 21 months sampling post storage, and demonstrates advantages in ease of use.
Acknowledgements
The authors would like to thank Vidya Keshav for assistance with editing of the manuscript, Caitlin Dixon and Jonathan Tsoka for data analysis, Lyndel Singh and the Clinical Laboratory Services Tuberculosis laboratory staff for assistance with laboratory testing.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
A.D. carried out the experiment. L.E.S. and A.D. conceived the original idea. L.E.S. supervised the project. A.D. took the lead in writing the manuscript. A.D., L.E.S., P.D.S., E.M. and W.S.S. provided critical feedback and helped shape the research, analysis and manuscript.
Sources of support
United Kingdom Medical Research Council, with funds received from the United Kingdom Government's Newton Fund under the United Kingdom/South Africa Newton Fund grant 015NEWTON TB (W.S.S. and L.E.S.) and the Bill & Melinda Gates Foundation grant OPP1171455 (W.S.S., L.E.S. and A.D.).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Disclaimer
The views expressed in the submitted article are those of the authors and not an official position of the institution or funder.
References
1.Molecular line probe assays for rapid screening of patients at risk of multi-drug resistant tuberculosis (MDR-TB) [homepage on the Internet]. World Health Organization; 2008 [cited 2022 May 14]. Available from: https://stoptb.org/assets/documents/about/cb/meetings/15/2.08-11%20Rolling%20out%20diagnostics%20in%20the%20field/2.08-11.2%20Line%20Probe%20Assays.pdf [ Links ]
2.World Health Organization (WHO) endorses new rapid tuberculosis test [homepage on the Internet]. World Health Organization; 2010 [updated 2010 Dec 08; cited 2022 Apr 07]. Available from: https://www.who.int/news/item/08-12-2010-who-endorses-new-rapid-tuberculosis-test [ Links ]
3.World Health Organization (WHO) meeting report of a technical expert consultation: Non-inferiority analysis of Xpert MTB/RIF ultra compared to Xpert MTB/RIF [homepage on the Internet]. World Health Organization; 2017 [updated 2017 Mar 24; cited 2022 Apr 07]. Available from: https://www.who.int/publications/i/item/WHO-HTM-TB-2017.04 [ Links ]
4.World Health Organization (WHO) endorses Truenat molecular diagnostic tests for TB [homepage on the Internet]. World Health Organization; 2020 [updated 2020 Jul 02; cited 2022 Apr 07]. Available from: https://www.finddx.org/newsroom/pr-02jul20/ [ Links ]
5.Module 3: Diagnostics, rapid diagnostic for tuberculosis detection, WHO consolidated guidelines on tuberculosis [homepage on the Internet]. World Health Organization; 2021 [updated 2021 Jul 07; cited 2022 Jul 07 2022]. Available from: https://www.who.int/publications/i/item/9789240029415 [ Links ]
6.Scott L, David A, Govender L, et al. Performance of the Roche Cobas MTB assay for the molecular diagnosis of pulmonary tuberculosis in a high HIV burden setting. J Mol Diagn. 2020;22(10):1225-1237. https://doi.org/10.1016/j.jmoldx.2020.06.018 [ Links ]
7.Hillemann D, Haasis C, Andres S, Behn T, Kranzer K. Validation of the FluoroType MTBDR Assay for detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2018;56(6):e00072-18. https://doi.org/10.1128/JCM.00072-18 [ Links ]
8.Wang SF, Ou XC, Zheng HW, Wang YF, Zhao YL. The Abbott RealTime MTB assay and the Cepheid GeneXpert assay show comparable performance for the detection of Mycobacterium tuberculosis in sputum specimens. Int J Infect Dis. 2016;45:78-80. [ Links ]
9.Chen JH, She KK, Kwong TC, et al. Performance of the new automated Abbott RealTime MTB assay for rapid detection of Mycobacterium tuberculosis complex in respiratory specimens. Eur J Clin Microbiol Infect Dis. 2015;34(9):1827-1832. https://doi.org/10.1007/s10096-015-2419-5 [ Links ]
10.Shah M, Paradis S, Betz J, et al. Multicenter study of the accuracy of the BD MAX™ MDR-TB assay for detection of Mycobacterium tuberculosis complex and mutations associated with resistance to Rifampin and Isoniazid. Clin Infect Dis. 2019;71(5):1161-1167. https://doi.org/10.1093/cid/ciz932 [ Links ]
11.Pipeline Report 2022. Treatment action group [homepage on the Internet]. 2022 [cited 2022 Nov 26]. Available from: https://www.treatmentactiongroup.org/resources/pipeline-report/2022-pipeline-report/ [ Links ]
12.Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: An overview in year 3 of the End TB era. Lancet Respir Med. 2018;6(4):299-314. https://doi.org/10.1016/S2213-2600(18)30057-2 [ Links ]
13.Boehme CC, Nabeta P, Henostroza G, et al. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J Clin Microbiol. 2007;45(6):1936-1940. https://doi.org/10.1128/JCM.02352-06 [ Links ]
14.Zhang M, Ren W, Sun X, et al. GeneChip analysis of resistant Mycobacterium tuberculosis with previously treated tuberculosis in Changchun. BMC Infect Dis. 2018;18(1):234. https://doi.org/10.1186/s12879-018-3131-8 [ Links ]
15.Nguyen TNA, Anton-Le Berre V, Bañuls A-L, Nguyen TVA. Molecular diagnosis of drug-resistant tuberculosis; A literature review. Front Microbiol. 2019;10:794. https://doi.org/10.3389/fmicb.2019.00794 [ Links ]
16.Igarashi Y, Chikamatsu K, Aono A, et al. Laboratory evaluation of the Anyplex™ II MTB/MDR and MTB/XDR tests based on multiplex real-time PCR and melting-temperature analysis to identify Mycobacterium tuberculosis and drug resistance. Diagn Microbiol Infect Dis. 2017;89(4):276-281. [ Links ]
17.Yoo I, Huh HJ, Kang OK, Jhun BW, Koh WJ, Lee NY. Advantages of the AdvanSure MDR-TB GenoBlot assay containing disputed rpoB mutation-specific probes in a routine clinical laboratory setting. Respir Med. 2019;146:71-75. https://doi.org/10.1016/j.rmed.2018.12.001 [ Links ]
18.Mbelele PM, Mohamed SY, Sauli E, et al. Meta-narrative review of molecular methods for diagnosis and monitoring of multidrug-resistant tuberculosis treatment in adults. Int J Mycobacteriol. 2018;7(4):299-309. https://doi.org/10.4103/ijmy.ijmy_135_18 [ Links ]
19.Kubica GP, Kim TH. Long-term preservation and storage of mycobacteria. Appl Microbiol. 1972;24(3):311-317. https://doi.org/10.1128/am.24.3.311-317.1972 [ Links ]
20.Huang TS, Chen YS, Lee SS, Tu HZ, Liu YC. Preservation of clinical isolates of Mycobacterium tuberculosis complex directly from MGIT culture tubes. Ann Clin Lab Sci. 2005;35(4):455-458. [ Links ]
21.GLI: Advancing TB diagnosis. 2014. Mycobacteriology laboratory manual [homepage on the Internet]. 2014 [cited 2022 Nov 26]. Available from: https://stoptb.org/wg/gli/assets/documents/gli_mycobacteriology_lab_manual_web.pdf [ Links ]
22.Metcalfe JZ, Streicher E, Theron G, et al. Mycobacterium tuberculosis subculture results in loss of potentially clinically relevant heteroresistance. Antimicrob Agents Chemother. 2017;61(11):e00888-17. https://doi.org/10.1128/AAC.00888-17 [ Links ]
23.Hanekom M, Streicher E, Van de Berg D, et al. Population structure of mixed Mycobacterium tuberculosis infection is strain genotype and culture medium dependent. PLoS One. 2013;8(7):e70178. https://doi.org/10.1371/journal.pone.0070178 [ Links ]
24.Kremer K, Van der Laan T, Van Soolingen D. Storage of mycobacterial strains. Methods Mol Med. 2001(54):359-365. https://doi.org/10.1385/1-59259-147-7:359 [ Links ]
25.Giampaglia CMS, De Brito AC, Martins MC, et al. Maintenance of Mycobacterium tuberculosis on glass beads. Ann Clin Lab Sci. 2009;39(1):51-54. [ Links ]
26.Baker M, Jeffries P. Use of commercially available cryogenic vials for long-term preservation of dermatophyte fungi. J Clin Microbiol. 2006;44(2):617-618. [ Links ]
27.Lakshman DK, Singh V, Camachoc ME. Long-term cryopreservation of non-spore-forming fungi in Microbank™ beads for plant pathological investigations. J Microbiol Methods. 2018;148:120-126. https://doi.org/10.1016/j.mimet.2018.04.007 [ Links ]
28.Espinel-Ingroff A, Montero D, Martin-Mazuelos E. Long-term preservation of fungal isolates in commercially prepared cryogenic microbank vials. J Clin Microbiol. 2004;42(3):1257-1259. [ Links ]
29.Pro Lab Diagnostics. Microbank [homepage on the Internet]. 2016 [n.d.; cited 2021 Nov 29]. Available from: https://www.pro-lab.com/wp-content/uploads/2016/11/PL170_Microbank_English.pdf [ Links ]
30.Siddiqi S, Rusch-Gerdes S. MGIT procedure manual [homepage on the Internet]. 2006 [cited 2021 May 24]. Available from: https://www.finddx.org/wp-content/uploads/2016/02/mgit_manual_nov2006.pdf [ Links ]
31.Kubica GP, Gontijo-Filho PP, Kim T. Preservation of mycobacteria at -70°C: Persistence of key differential features. J Clin Microbiol. 1977;6(2):149-153. https://doi.org/10.1128/jcm.6.2.149-153.1977 [ Links ]
32.Svensson RJ, Sabiiti W, Kibiki GS, et al. Model-based relationship between the molecular bacterial load assay and time-to-positivity in liquid culture. Antimicrob Agents Chemother. 2019;63(10):e00652-19. https://doi.org/10.1128/AAC.00652-19 [ Links ]
33.Chirenda J, Chipinduro M, De Kock M, et al. Recovery of Mycobacterium tuberculosis from positive mycobacterium growth indicator tubes stored at room temperature for up to 6 years in lowincome and hightuberculosisburden country. Int J Mycobacteriol. 2019;8:185-189. https://doi.org/10.4103/ijmy.ijmy_46_19 [ Links ]
34.Van Soolingen D, Van der Laan T, Kremer K. Storage of mycobacterial strains [homepage on the Internet]. 2018 [cited 2023 Jun 25]. Available from: www.rivm.nl/sites/default/files/2018-11/protocol%20storage%20of%20mycobacteria%20a1a.pdf [ Links ]
35.Kim S. Drug-susceptibility testing in tuberculosis: Methods and reliability of results. Eur Respir J. 2005;25(3):564-569. https://doi.org/10.1183/09031936.05.00111304 [ Links ]
36.Global Laboratory Initiative. Isolate storage, packaging and transportation [homepage on the Internet]. [cited 2022 Nov 26]. Available from: https://stoptb.org/wg/gli/assets/documents/srt/MSH%20Rwanda%20-%20Isolate%20Storage%20Packaging%20and%20Transportation.pdf [ Links ]
37.International Air Transport Assoication (IATA). Dangerous goods regulation. IATA guidelines. 60th ed. 2019. Montreal: IATA. [ Links ]
 Correspondence:
Correspondence:
Anura David
anura.david@witshealth.ac.za
Received: 28 Jan. 2023
Accepted: 23 Aug. 2023
Published: 25 Oct. 2023
Note: Additional supporting information may be found in the online version of this article as Online Supplementary Document 1.














