Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Journal of Laboratory Medicine
On-line version ISSN 2225-2010
Print version ISSN 2225-2002
Afr. J. Lab. Med. vol.12 n.1 Addis Ababa 2023
http://dx.doi.org/10.4102/ajlm.v12i1.2065
ORIGINAL RESEARCH
Seroprevalence of SARS-CoV-2 immunoglobulin G in HIV-positive and HIV-negative individuals in KwaZulu-Natal, South Africa
Kerri-Lee A. FrancoisI, II; Nokukhanya MsomiI, II; Kerusha GovenderI, II; Lilishia GounderI, II; Pravi MoodleyI, II; Raveen ParboosingI, II, III; Indrani ChettyIV; Lunga XabaIV; Aabida KhanI, II
IDiscipline of Virology, Faculty of Health Sciences, School of Laboratory Medicine and Medical Sciences, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IINational Health Laboratory Services, Inkosi Albert Luthuli Central Hospital, Durban, South Africa
IIIDepartment of Medical Virology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVDiscipline of Virology and National Health Laboratory Service, Inkosi Albert Luthuli Central Hospital, Durban, South Africa
ABSTRACT
BACKGROUND: KwaZulu-Natal ranked second highest among South African provinces for the number of laboratory-confirmed cases during the second wave of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. The seroprevalence of SARS-CoV-2 among certain vulnerable groups, such as people living with HIV in KwaZulu-Natal, is unknown.
OBJECTIVE: The study aimed to determine the prevalence of SARS-CoV-2 immunoglobulin G (IgG) in HIV-positive versus HIV-negative patients.
METHODS: This was a retrospective analysis of residual clinical blood specimens unrelated to coronavirus disease 2019 (COVID-19) submitted for diagnostic testing at Inkosi Albert Luthuli Central Hospital, Durban, from 10 November 2020 to 09 February 2021. Specimens were tested for SARS-CoV-2 immunoglobulin G on the Abbott Architect analyser.
RESULTS: A total of 1977/8829 (22.4%) specimens were positive for SARS-CoV-2 antibodies. Seroprevalence varied between health districts from 16.4% to 37.3%, and was 19% in HIV-positive and 35.3% in HIV-negative specimens. Seroprevalence was higher among female patients (23.6% vs 19.8%; p < 0.0001) and increased with increasing age, with a statistically significant difference between the farthest age groups (< 10 years and > 79 years; p < 0.0001). The seroprevalence increased from 17% on 10 November 2020 to 43% on 09 February 2021 during the second wave.
CONCLUSION: Our results highlight that during the second COVID-19 wave in KwaZulu-Natal a large proportion of people living with HIV were still immunologically susceptible. The reduced seropositivity in people with virological failure further emphasises the importance of targeted vaccination and vaccine response monitoring in these individuals.
WHAT THE STUDY ADDS: This study contributes to data on SARS-CoV-2 seroprevalence before and during the second wave in KwaZulu-Natal, South Africa, which has the highest HIV prevalence globally. Reduced seropositivity was found among people living with HIV with virological failure, highlighting the importance of targeted booster vaccination and vaccine response monitoring.
Keywords: SARS-CoV-2 IgG prevalence; seroprevalence HIV-positive and HIV-negative; anti-SARS-CoV-2 IgG HIV-positive and negative; seroprevalence SARS-CoV-2 IgG; KwaZulu-Natal.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is one of the most significant pandemics in history. In Africa, South Africa has the highest number of confirmed cases (Africa CDC COVID-19 Dashboard, 2021 - https://africacdc.org/covid-19/). KwaZulu-Natal province ranked second-highest nationally for the number of laboratory-confirmed cases during the second wave of the SARS-CoV-2 epidemic in South Africa.1
The first wave in KwaZulu-Natal was around June 2020 - July 2020. The wave was followed by lower but sustained viral circulation between August 2020 and October 2020 and a more explosive second wave from November 2020 to February 2021. The second wave was of greater magnitude than the first, with the emergence of the more transmissible beta variant.2,3 The third wave of the epidemic in South Africa started in May 2021, with a resurgence in KwaZulu-Natal in June 2021. The more transmissible delta variant rapidly displaced the beta variant between May 2021 and August 2021, while the even more highly transmissible omicron variant overtook the delta variant between November 2021 and January 2022.2,4
Polymerase chain reaction (PCR) is most accurate for the diagnosis of acute SARS-CoV-2 infection.5 However, the use of PCR-confirmed cases for reporting may underestimate the true extent of the SARS-CoV-2 pandemic. Although not indicated for the diagnosis of acute infection, serology may give a more accurate reflection of the true prevalence of infection in people who may not have had a PCR test during acute infection or who were asymptomatic.6
Even though South Africa has passed the third, fourth and fifth waves of the SARS-CoV-2 epidemic and is currently experiencing a transition phase, serological surveillance remains a vital tool to understand the true extent of SARS-CoV-2 exposure and inform public health responses.7
Seroprevalence in 2020 was estimated to be 5-10 times higher than reported cases identified by PCR.8,9,10 A population-based seroepidemiological survey in Gauteng province in South Africa, that started 8 weeks into the first wave and ended at the peak of the second wave, estimated that there were 2.89 million SARS-CoV-2 infections, which is 7-8 times higher than the reported 332 000 PCR cases.10 Local SARS-CoV-2 seroprevalence rates were over 60% in black South African blood donors11 and 30% - 40% among patients accessing care during the first wave in the Cape Town Metropolitan sub-districts.12 Similarly, seroprevalence studies in Spain, Geneva and New York reported seroprevalence higher than the number of reported cases.13,14,15
Ideally, population-based serosurveys should be used to estimate seroprevalence nationally.16 However, serosurveys require extensive resources, and involve active recruitment and prospective follow-up of individuals. Using residual clinical specimens submitted for either routine screening or diagnostic testing could provide a snapshot16,17 and is a convenient way to conduct passive surveillance. In South Africa, seroprevalence studies using convenience specimens have been conducted by the South African Blood Bank Services (SANBS) in the Western Cape and Gauteng.11,12,18
KwaZulu-Natal has the world's highest HIV prevalence19 and has the second highest number of reported SARS-CoV-2 infections nationally. Second-wave infections in South Africa caused by the beta variant were associated with increased disease severity, especially in people living with HIV20 when no vaccines were available. Thus, an accurate estimate of SARS-COV-2 seroprevalence among people living with HIV was important in the early phase of the SARS-CoV-2 epidemic for outbreak surveillance, implementation of vaccination strategies, and to assess the effectiveness of non-pharmaceutical interventions in KwaZulu-Natal. We therefore determined the SARS-CoV-2 immunoglobulin G (IgG) seroprevalence among people with HIV infection in KwaZulu-Natal.
Methods
Ethical considerations
This study was approved by University of KwaZulu-Natal Biomedical Research Ethics Committee (Ref. BCA256/010). Informed patient consent was not required as this was a retrospective study using residual diagnostic specimens. No additional specimens were collected. All residual clinical specimens were de-identified and labelled with a unique study number; patient privacy and confidentiality data were protected in accordance with the Declaration of Helsinki. Data were collected in a password-protected Excel spreadsheet (Microsoft Corp., Redmond, Washington, United States) on a dedicated computer behind firewall-protected servers, which was only accessible to the primary investigator.
Study design
The authors retrospectively tested residual clinical sera and plasma specimens submitted to the Department of Virology at Inkosi Albert Luthuli Central Hospital, Durban, for diagnostic testing unrelated to COVID-19 from 10 November 2020 to 09 February 2021, representing the period before and during the second wave (22 November 2020 to 27 March 2021) of the SARS-CoV-2 epidemic in KwaZulu-Natal.
Sample size
A minimum sample size of 7294 was estimated using OpenEpi (Version 3.1; http://www.openepi.com/)21 based on a SARS-CoV-2 prevalence of 5% to 10% before the second wave peak with a maximum 1% margin error and using published seroprevalence data from studies done globally (5%,10 1% - 6.9%22 and 1% - 10%8), the assumed point prevalence was calculated.
Using the STATA® (StataCorp, College Station, Texas, United Staes) sampsi function, the sample size was calculated to compare SARS-CoV-2 seropositivity in HIV-positive and HIV-negative individuals with a power of 80%, two-sided alpha of 0.05 and equal size in each group. A sample size of 3682 would be sufficient to detect a 1% difference if the prevalence is low (1%) and a 3% difference if the prevalence is high (13%).8,13,22
Specimen selection
Sera and plasma specimens with sufficient volumes from the viral serology (HIV and hepatitis B enzyme-linked immunosorbent assay and HIV viral load laboratory sections) were selected for SARS-CoV-2 IgG testing. Specimens with inadequate volumes (< 100 µL) were excluded. The selected specimen results were linked to demographic information extracted from the laboratory information system. Specimens from the same patient were tested once only.
Laboratory methods
Testing for SARS-CoV-2 IgG was performed on the Abbott Architect using the Abbott Architect SARS-CoV-2 IgG assay (Abbott Diagnostics, Abbott Park, Illinois, United States).23 Sera and plasma specimens were stored at 2 °C - 8 °C for not more than 7 days and −20 °C for long-term (> 7 days) storage. The assay is an automated two-step chemiluminescent microparticle immunoassay for qualitative detection of SARS-CoV-2 IgG antibodies against the SARS-CoV-2 nucleocapsid protein. The assay was performed and interpreted according to the manufacturer's instructions.24
Statistical analysis
Statistical analysis was done using STATA® version 15 (StataCorp, College Station, Texas, United States). We performed a logistic regression analysis to determine whether age, gender, HIV seropositivity, and HIV viral load were associated with SARS-CoV-2 seropositivity, with significance level set to 0.05.
Results
A total of 9083 specimens were tested. However, 254 specimens were excluded from the analysis due to existing SARS-CoV-2 IgG test requests (n = 248) or mismatched laboratory information (n = 6). A total of 8829 (Table 1) specimen results were analysed: 5724 (64.8%) of the specimens were from female and 2865 (32.5 %) from male patients, while 240 (2.7%) had no specified gender; 1309 (14.8%) of sera specimens had HIV enzyme-linked immunosorbent assay results, and 5522 (62.5%) of plasma specimens had HIV viral load results.
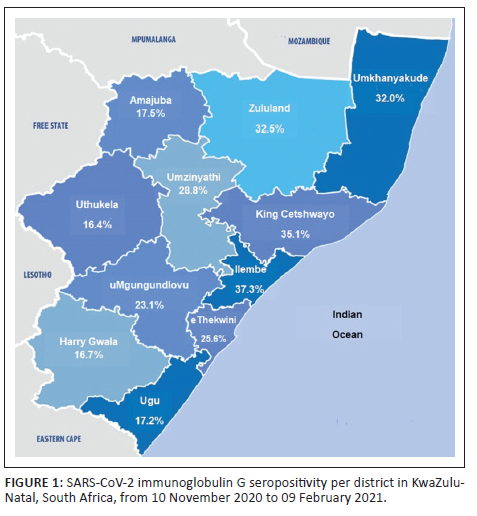
The average SARS-CoV-2 seropositivity across 11 health districts (Figure 1) during the second wave was 22.4%; the iLembe district had the highest seropositivity (37.3%), followed by King Cetshwayo (35.1%), and uMkhanyakude (32%). Weekly seropositivity increased with increasing SARS-CoV-2 cases (Figure 2), reaching 43% during the second wave in KwaZulu-Natal.
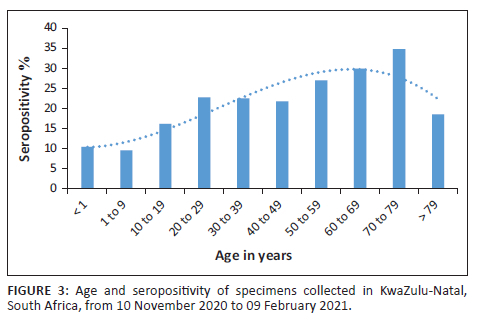
Seropositivity among female patients was 23.6% (odds ratio: 0.705, 95% confidence interval: 0.617-0.806) while male patients had lower odds of seropositivity (p < 0.0001) (Table 1). Seropositivity increased with increasing age, with a 1.012 increase in odds for every year increase in age (p < 0.0001) (Figure 3). Seropositivity was highest in the 70-79 year age group and lowest in those under 9 years of age. Seropositivity (Table 1) was higher in the HIV-negative compared to the HIV-positive (35.3% vs 19%; odds ratio: 0.402; 95% confidence interval: 0.349-0.463). HIV positivity with a viral load greater than 1000 copies/mL was associated with lower odds of SARS-CoV-2 seropositivity (odds ratio: 0.518; 95% confidence interval: 0.401-0.669). No statistically significant interaction (at a 10% level) was noted between HIV status and age or gender.
Discussion
We assessed the SARS-CoV-2 IgG seropositivity in convenience specimens submitted to the Department of Virology at Inkosi Albert Luthuli Central Hospital, Durban, before and during the peak of the second COVID-19 wave caused by the beta variant. We observed an increase in seropositivity from 17% before to 43% during the second wave in KwaZulu-Natal, reflective of the increase in the number of infected individuals during the second wave, with a mean SARS-CoV-2 seropositivity of 22.4% across the study period. There were statistically significant associations between SARS-CoV-2 seropositivity, age, gender, and HIV status. Our results indicate that the true extent of the SARS-CoV-2 epidemic in KwaZulu-Natal may have been underestimated due to reporting of PCR results alone. This is similar to other local seroprevalence studies that report higher seroprevalence compared to the number of reported PCR cases.10,11,12,25
The seropositivity in our study was lower than the seroprevalence reported by surveillance studies in Cape Town (40%) and Gauteng (27.8%).12,18 The lower prevalence may be because the specimens were not collected immediately after the first wave in KwaZulu-Natal; they were collected 4 months later, thus affecting the detection of IgG, which wanes over time. The sentinel surveillance studies were started directly after the first wave in both Cape Town and Gauteng.
Furthermore, the majority (63%) of the specimens in our study were from HIV-positive individuals; 10% of these specimens had an HIV viral load greater than 1000 copies/mL and reduced SARS-CoV-2 seropositivity. HIV viral load greater than 1000 copies/mL had a statistically significant association with lower odds of SARS-CoV-2 IgG positivity. HIV infection is associated with a reduced immune response to infectious pathogens.26 However, in individuals on antiretroviral therapy, immune responses to SARS-CoV-2 are reported to be similar to that in HIV-negative individuals.27,28 People on antiretroviral therapy may display immune reconstitution;26 however, chronic immune activation and impaired B-cell responses in individuals on antiretroviral therapy with virologic failure may result in reduced antibody responses26,28 and lower IgG concentrations,29 accounting for the lower seropositivity in those with HIV infection.
Lower SARS-CoV-2 IgG concentrations may affect the detectability of IgG,30 which may be lower than the detectable limit of the assay. Based on a local evaluation of the performance of the Abbott Architect SARS-CoV-2 IgG assay, sensitivity is highest 30-41 days after a positive PCR, following moderate to severe infection.23 However in our study reduced seropositivity was most likely due to reduced immunity in people with uncontrolled HIV and the timing of sampling in relation to the epidemic waves.23
We have demonstrated that SARS-CoV-2 seropositivity increased with age, with children under 10 years having the lowest seropositivity (11.1%). Similar patterns have been described locally25 and internationally.15
Our reported seropositivity (10%) in children under 10 years during the second wave in KwaZulu-Natal indicated that these children may have been more vulnerable to SARS-CoV-2 infection. However, the rate of infections in South Africa among these children was 1.4%,31 suggesting that PCR testing alone may have underestimated infection rates or that these children were asymptomatic. However, a seroprevalence in children under 9 years during the first wave in Gauteng was reported to be about 20%,18 most likely due to Gauteng having the highest infection rate nationally during the entire epidemic.1
We found no discernible pattern in the district seroprevalence during the second wave. However, the rate of infections in the Ethekwini, iLembe, Ugu, and Uthukela districts were subsequently lower in the third wave.1
Limitations
Firstly, the specimens tested were from individuals accessing public healthcare and thus may not represent the general population. However, it potentially represents people living with HIV accessing antiretroviral therapy in our province.
Secondly, surveillance using residual clinical specimens without exposure time data, as done in this study, may underestimate seropositivity.32 Thirdly, seropositivity may be underestimated since SARS-CoV-2 IgG detection and durability is directly proportional to disease severity.33,34 Although antibody positivity was still high in another study at 8 months after mild and asymptomatic infection,35 overall detectability and durability may be reduced in HIV-positive persons.36
Fourthly, the Abbott Architect SARS-CoV-2 IgG assay detects IgG to nucleocapsid antigens only and at the time was the only approved commercial serological assay by the South African Health Products Regulatory Authority.37
The use of nucleocapsid IgG solely to determine seroprevalence may underestimate the true rate of past infections.32 Nucleocapsid IgG declines more rapidly than spike IgG. However, nucleocapsid IgG is more sensitive within the first 14 days of SARS-CoV-2 infection as compared to spike IgG.34 Lastly, antibody cross-reactivity with other Betacoronaviruses may lead to false positives.23,38 However, the Abbott Architect SARS-CoV-2 IgG has a specificity of 99%.23,38
Conclusion
Our results highlight that after the second wave in KwaZulu-Natal, many people accessing public healthcare were immunologically susceptible. Furthermore, reduced seropositivity in HIV-positive individuals with HIV viral loads greater than 1000 copies/mL further emphasised the importance of targeted vaccination of people living with HIV and monitoring response to vaccination in these individuals.39,40
Acknowledgements
The authors would like to acknowledge and thank the staff from the Department of Virology at Inkosi Albert Luthuli Central Hospital for their assistance and dedication to the research project, as well as the National Health Laboratory Service for providing the kits used for this research.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
K.-L.A.F., N.M., K.G., L.G., P.M., R.P., I.C., L.X. and A.K. contributed to the conceptualisation, ethics application, writing of the manuscript, data collection and analysis, and have read and agreed to the published version of the manuscript.
Sources of support
This research did not receive a grant from any funding agency in the public, commercial, or non-profit sectors. We would like to express gratitude to the National Health Laboratory Service for providing kits used for this research.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any of the affiliated institutions of the authors.
References
1.Bapela MP, Ngcobo A, Sosibo C, Mhlongo B. KwaZulu-Natal Department of Health COVID-19 Situation report. 9 June 2022. Durban: DoH; 2022. [ Links ]
2.Abdool Karim SS, Baxter C. Impact of SARS-CoV-2 variants of concern on COVID-19 epidemic in South Africa. Trans R Soc S Afr. 2022;77(1):101-104. https://doi.org/10.1080/0035919X.2021.2011801 [ Links ]
3.Tegally H, Wilkinson E, Giovanetti M, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592(7854):438-443. https://doi.org/10.1038/s41586-021-03402-9 [ Links ]
4.Subramoney K, Mtileni N, Bharuthram A, et al. Identification of SARS-CoV-2 Omicron variant using spike gene target failure and genotyping assays, Gauteng, South Africa, 2021. J Med Virol. 2022;94(8):3676-3684. https://doi.org/10.1002/jmv.27797 [ Links ]
5.Burgess S, Ponsford MJ, Gill D. Are we underestimating seroprevalence of SARS-CoV-2? London: British Medical Journal Publishing Group; 2020. [ Links ]
6.Alter G, Seder R. The power of antibody-based surveillance. N Engl J Med. 2020;383(18):1782-1784. [ Links ]
7.Mayne E, Scott L, Semete B, et al. The role of serological testing in the SARS-CoV-2 outbreak. S Afr Med J. 2020;110(9):842-845. https://doi.org/10.7196/SAMJ.2020.v110i9.15098 [ Links ]
8.Byambasuren O, Dobler CC, Bell K, et al. Comparison of seroprevalence of SARS-CoV-2 infections with cumulative and imputed COVID-19 cases: Systematic review. PloS One. 2021;16(4):e0248946. [ Links ]
9.Rostami A, Sepidarkish M, Leeflang MMMG, et al. SARS-CoV-2 seroprevalence worldwide: A systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(3):331-340. https://doi.org/10.1016/j.cmi.2020.10.020 [ Links ]
10.Mutevedzi PC, Kawonga M, Kwatra G, et al. Estimated SARS-CoV-2 infection rate and fatality risk in Gauteng Province, South Africa: A population-based seroepidemiological survey. Int J Epidemiol. 2021;51(2):404-417. https://doi.org/10.1093/ije/dyab217 [ Links ]
11.Sykes W, Mhlanga L, Swanevelder R, et al. Prevalence of anti-SARS-CoV-2 antibodies among blood donors in Northern Cape, KwaZulu-Natal, Eastern Cape, and Free State provinces of South Africa in January 2021. Research Square. 2021; Preprint. https://doi.org/10.21203/rs.3.rs-233375/v1 [ Links ]
12.Hsiao M, Davies M, Kalk E. SARS-CoV-2 seroprevalence in the Cape Town Metropolitan sub-districts after the peak of infections. NICD COVID-19 Special Public Health Surveill Bull. 2020;18:1-9. [ Links ]
13.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535-544. https://doi.org/10.1016/S0140-6736(20)31483-5 [ Links ]
14.Alfego D, Sullivan A, Poirier B, Williams J, Adcock D, Letovsky S. A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States. EclinicalMedicine. 2021;36:100902. https://doi.org/10.1016/j.eclinm.2021.100902 [ Links ]
15.Pagani G, Conti F, Giacomelli A, et al. Seroprevalence of SARS-CoV-2 significantly varies with age: Preliminary results from a mass population screening. J Infect. 2020;81(6):e10. https://doi.org/10.1016/j.jinf.2020.09.021 [ Links ]
16.Chen X, Chen Z, Azman AS, et al. Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis. Lancet Glob. Health. 2021;9(5):e598-e609. [ Links ]
17.Cheng MP, Yansouni CP, Basta NE, et al. Serodiagnostics for Severe Acute Respiratory Syndrome-Related Coronavirus 2: A narrative review. Ann Intern Med. 2021;173(6):450-460. [ Links ]
18.George JA, Khoza S, Mayne E, et al. Sentinel seroprevalence of SARS-CoV-2 in Gauteng Province, South Africa, August-October 2020. South Afr Med J. 2021;111(11):1078-1083. [ Links ]
19.Simbayi LCZK, Zuma K, Zungu N, et al. South African National HIV prevalence, incidence, behaviour and communication survey, 2017: Towards achieving the UNAIDS 90-90-90 targets. Cape Town: HSRC Press; 2019. [ Links ]
20.Jassat W, Mudara C, Ozougwu L, et al. Difference in mortality among individuals admitted to hospital with COVID-19 during the first and second waves in South Africa: A cohort study. Lancet Glob. Health. 2021;9(9):e1216-e1225. https://doi.org/10.1101/2021.03.09.21253184 [ Links ]
21.Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health. 2013 [cited 2020 Jul 29]. Available from: http://www.OpenEpi.com [ Links ]
22.Havers FP, Reed C, Lim T, et al. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020;180(12):1576-1586. https://doi.org/10.1001/jamainternmed.2020.4130 [ Links ]
23.Jugwanth S, Gededzha MP, Mampeule N, et al. Performance of the Abbott SARS-CoV-2 IgG serological assay in South African 2 patients. PloS One. 2022;17(2):e0262442. https://doi.org/10.1371/journal.pone.0262442 [ Links ]
24.SARS-CoV Abbott Architect. IgG instructions for use. H07943R01. Available from: https://www.corelaboratory.abbott/int/en/home [ Links ]
25.Kleynhans J, Tempia S, Wolter N, et al. SARS-CoV-2 seroprevalence in a rural and urban household cohort during first and second waves of infections, South Africa, July 2020-March 2021. Emerg Infect Dis. 2021;27(12):3020-3029. https://doi.org/10.3201/eid2712.211465 [ Links ]
26.Moir S, Fauci AS. B-cell responses to HIV infection. Immunol Rev. 2017;275(1):33-48. https://doi.org/10.1111/imr.12502 [ Links ]
27.Pallikkuth S, Sharkey M, Beauchamps L, et al. Persistence of SARS-CoV-2-specific Ab response in HIV+ individuals on ART. Top Antivir Med. 2021:88-88. [ Links ]
28.Ambrosioni J, Blanco JL, Reyes-Urueña JM, et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV. 2021;8(5):e294-e305. https://doi.org/10.1016/S2352-3018(21)00070-9 [ Links ]
29.Spinelli MA, Lynch KL, Yun C, et al. SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: A matched case-control observational study. Lancet HIV. 2021;8(6):e334-e341. https://doi.org/10.1016/S2352-3018(21)00072-2 [ Links ]
30.Spinelli M, Lynch K, Yun C, et al. SARS-CoV-2 seroprevalence and IgG levels are lower among people living with HIV. Top Antiviral Med. 2021:242-242. [ Links ]
31.National Institute for Communicable Diseases (NICD). COVID-19 in children surveillance report. 2021. Available from: https://www.nicd.ac.za/wp-content/uploads/2021/09/COVID-19-in-children-surveillance-report.pdf. [ Links ]
32.Fenwick C, Croxatto A, Coste AT, et al. Changes in SARS-CoV-2 spike versus nucleoprotein antibody responses impact the estimates of infections in population-based seroprevalence studies. J Virology. 2021;95(3):e01828-20. [ Links ]
33.Paleker M, Tembo Y, Davies M, et al. Asymptomatic COVID-19 - implications for the control of transmission in South Africa. Public Health Action. 2021;11(2):58-60. https://doi.org/10.5588/pha.20.0069. [ Links ]
34.Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med. 2020;217(6):e20200678. https://doi.org/10.1084/jem.20200678 [ Links ]
35.Choe PG, Kim K-H, Kang CK, et al.Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection. Emerg Infect Dis. 2021;27(3):928. https://doi.org/10.3201/eid2703.204543 [ Links ]
36.Brown LB, Spinelli MA, Gandhi M. The interplay between HIV and COVID-19: Summary of the data and responses to date. Curr Opin HIV AIDS. 2021;16(1):63-73. https://doi.org/10.1097/COH.0000000000000659 [ Links ]
37.South African Health Products Regulatory Authority. SAHPRA approves SARS-CoV-2 serology test kits [press release]. 25 August 2020. [ Links ]
38.Ng KW, Faulkner N, Cornish GH, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370(6522):1339-1343. https://doi.org/10.1126/science.abe1107 [ Links ]
39.Hassold N, Brichler S, Ouedraogo E, et al. Impaired antibody response to COVID-19 vaccination in advanced HIV infection. AIDS. 2022;36(4):F1-F5. https://doi.org/10.1097/QAD.0000000000003166 [ Links ]
40.Xu X, Vesterbacka J, Aleman S, Nowak P. High seroconversion rate after vaccination with mRNA BNT162b2 vaccine against SARS-CoV-2 among people with HIV - But HIV viremia matters? AIDS. 2022;36(3):479-481. https://doi.org/10.1097/QAD.0000000000003135 [ Links ]
 Correspondence:
Correspondence:
Kerri-Lee Francois
francoisk@ukzn.ac.za
Received: 26 Aug. 2022
Accepted: 29 Mar. 2023
Published: 29 June 2023














