Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
African Journal of Laboratory Medicine
versión On-line ISSN 2225-2010
versión impresa ISSN 2225-2002
Afr. J. Lab. Med. vol.4 no.1 Addis Ababa 2015
http://dx.doi.org/10.4102/ajlm.v4i1.178
ORIGINAL RESEARCH
Potential for false-positive HIV test results using rapid HIV testing algorithms
Rosemary A. AuduI; Rosemary N. OkoyeII; Chika K. OnwuamahI; Fehintola A. IgeI; Adesola Z. MusaIII; Nkiruka N. OdunukweIV; Daniel I. OnwujekweIV; Oliver C. EzechiIV; Emmanuel O. IdigbeI; Phyllis J. KankiV
IHuman Virology Laboratory, Nigerian Institute of Medical Research, Lagos, Nigeria
IIClinical Diagnostic Laboratory, Nigerian Institute of Medical Research, Lagos, Nigeria
IIIMonitoring and Evaluation Unit, Nigerian Institute of Medical Research, Lagos, Nigeria
IVClinical Sciences Division, Nigerian Institute of Medical Research, Lagos, Nigeria
VHarvard School of Public Health, Boston, Massachusetts, United States
ABSTRACT
BACKGROUND: In order to scale up access to HIV counselling and testing in Nigeria, an HIV diagnostic algorithm based on rapid testing was adopted. However, there was the need to further evaluate the testing strategy in order to better assess its performance, because of the potential for false positivity
OBJECTIVES: The objective of this study was to compare positive HIV test results obtained from the approved rapid testing algorithm with results from western blot tests performed on samples from the same patient
METHODOLOGY: A retrospective review was conducted of HIV screening and confirmatory results for patients seen between 2007 and 2008. Rapid test and western blot results were extracted and compared for concordance. Discordant results were further reviewed using a combination of HIV-1 RNA viral load and CD4+ cell count test results and clinical presentation from medical records
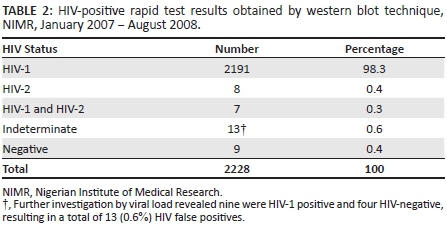
RESULTS: Analysis of 2228 western blot results showed that 98.3% (n = 2191) were positive for HIV-1, 0.4% (n = 8) were positive for HIV-2 and 0.3% (n = 7) were dual infections (positive for both HIV-1 and HIV-2); 0.6% (n = 13) were indeterminate and 0.4% (n = 9) were negative. Further investigation of the 13 indeterminate results showed nine to be HIV-1 positive and four to be HIV-negative, for a total of 13 negative results. The positive predictive value of the HIV counselling and testing algorithm was 99.4%
CONCLUSION: Using the rapid testing algorithm alone, false positives were detected. Therefore, effective measures such as training and retraining of staff should be prioritised in order to minimise false-positive diagnoses and the associated potential for long-term psychological and financial impact on the patients
Introduction
The diagnosis of HIV infection is most often based on the detection of serum antibodies to HIV. These serological tests could be classified either as screening tests, such as rapid tests or enzyme linked immuno-sorbent assay (ELISA), or confirmatory tests, such as western blot (WB). Prior to the availability of rapid testing, same-day results were not obtainable and an estimated one third of individuals tested did not return to learn their HIV status.1 Compared with ELISA and WB, rapid test assays are cheaper, easier to perform and the results are readily available on the same day. In 1999, the World Health Organization and the Joint United Nations Programme on HIV/AIDS recommended the use of a combination of rapid test assays or ELISAs to confirm positive results, employing a highly sensitive test as the first screening test and a second, highly specific confirmatory test to verify the detection of antibodies specific to HIV. This was considered to be as reliable for confirmation as WB but at a much lower cost.2
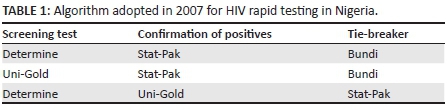
In 2007, the government of Nigeria adopted a serial testing algorithm with three combinations of the following rapid test assays: Determine® (Abbott, Tokyo, Japan), Stat-Pak® (Chembio Diagnostic Systems, Medford, New York, United States) or Uni-Gold™ (Trinity Biotech, Jamestown, New York, United States). Samples with discordant results were further tested with Bundi RT (BUNDI International Diagnostics Ltd, Aba, Nigeria) as the tiebreaker.3 The adopted algorithm could be used in any of the combinations shown in Table 1. Because of quality issues with the Bundi test kits, the use of the kit was suspended; the third combination was retained and is currently in use in the country.
As with all screening assays, HIV rapid test screening has a certain degree of false-positive results, irrespective of the algorithm in use.4 For this reason, a confirmatory test is necessary for a definitive diagnosis. Evaluations of HIV rapid testing strategies have been reported in some African countries where the results have been used to formulate alternative HIV testing strategies.5,6 Regardless of which rapid test algorithm is used, false-positive results may still occur;7,8 1% - 2% of reactive rapid test results have been found to be negative with an HIV nucleic acid test.7 Since the introduction of the 2007 HIV rapid test strategy in Nigeria, its performance has not been evaluated. Therefore, the assessment of the algorithm's performance remains an important priority to better inform national policy and ensure accurate HIV diagnosis and surveillance. The aim of this study was to compare positive HIV test results obtained from the approved rapid testing algorithm with results from WB tests performed on samples from the same patient.
Methods
Study setting
The study was conducted at the Nigerian Institute of Medical Research (NIMR), Lagos, a parastatal of the Federal Ministry of Health. NIMR has an HIV treatment centre that currently provides comprehensive HIV care, antiretroviral treatment (ART) and support for over 18 000 patients. The centre is supported by the Government of Nigeria and the US President's Emergency Plan for AIDS Relief-funded programme for ART; as a result, all services are provided free to patients. Patients who screen positive for HIV at the HIV Counselling and Testing (HCT) centre or any government of Nigeria-approved HIV counselling and testing centre are referred to the treatment programme for further management. These patients are clinically assessed and sent to the NIMR virology laboratory for baseline laboratory assessment. The NIMR virology laboratory is an ISO 9001:2008 certified laboratory and employs well trained and competent personnel to perform the laboratory assays. Quality assurance measures undertaken by the laboratory include: participation in external quality assessment for all assays; equipment maintenance; and several measures to verify pre-analytical, analytical and post-analytical processes.
National testing algorithm
The approved national serial testing algorithm used for this study was: Determine, Unigold and Stat-Pak as tie breaker. This algorithm is currently in use in Nigeria; thus, the results of this study are relevant to the current situation in the country.
Study design
This was a retrospective study, which analysed data from the programme's electronic database. Cases with discordant results were further reviewed using a combination of plasma viral load, CD4+ cell counts and clinical data to determine a presumptive HIV diagnosis or progression of infection. HIV test results generated between January 2007 and August 2008 were analysed. Discordant results were reviewed until December 2011.
Data abstraction and inclusion criteria
Data were abstracted from records of patients aged 18 years or older who had tested positive for HIV using the 2007 Nigerian rapid test algorithm at the NIMR HCT, satellite sites or private clinics. Data for patients sent to the NIMR virology laboratory for confirmation of HIV diagnosis by WB were abstracted for this study. Data from all patients who had given informed consent to participate in research studies were included. WB test result data included all positive, negative and indeterminate results. Indeterminate results were defined as reactivity profiles that were not compatible with either a positive or a negative interpretation.
Laboratory methods
The NIMR HCT site conducted HIV testing using the national testing algorithm; this required the use of non-cold chain dependent rapid test kits supplied by the government of Nigeria through the Central Medical Stores. The satellite sites and private clinics who referred HIV-positive patients for care and treatment also indicated the rapid test kits used in obtaining the positive HIV results. At the NIMR virology laboratory, the baseline assessment included: HIV confirmation using the WB technique (Immunetics Inc., Boston, Massachusetts, United States) to detect specific viral proteins for both HIV-1 and HIV-2; CD4+ cell count (cells/µL) using the flow cytometry technique employed by the Partec Cyflow Counter II (Gorlitz, Germany) to determine eligibility for the initiation of ART; and quantitation of HIV-1 RNA viral load in plasma (Roche Amplicor v1.5, Mannheim, Germany) with a lower limit of detection of 400 copies/mL. The viral load assay was further used to monitor the effectiveness of therapy and for timely identification of poor adherence and possible virologic failure.
Statistical analyses
The positive predictive value was calculated based on the results of the rapid tests and WB using standard formulae. Exact 95% confidence intervals for these proportions were calculated. Analyses were conducted using Statistical Package for Social Sciences version 17 for Windows (IBM Corporation, Chicago, Illinois, United States).
Ethical considerations
Ethical approval for this study was obtained from the NIMR and Harvard School of Public Health Institutional Review Boards. Written informed consent (dated 14 November 2006) was obtained prior to enrolment of patients for both services and research.
Results
Results from 2228 different patients meeting the inclusion criteria were extracted from the programme's electronic database. There was a male: female ratio of 1.9:1 and the mean age was 38.8 ± 9.0 years. The NIMR HCT facility accounted for 967 (43.4%) of the results abstracted, whereas 1261 (56.6%) were from satellite sites and private clinics. The analyses of the WB results showed that 98.3% were positive for HIV-1, 0.4% were positive for HIV-2 and 0.3% had HIV-1 and HIV-2 dual infections. There were also 13 (0.6%) indeterminate and nine (0.4%) negative results (Table 2). The 13 indeterminate results were further investigated using plasma viral load levels and nine (69.2%) were found to be infected with HIV-1 based on very high HIV-1 viral load levels (median = 310 377 RNA copies/mL; range = 2150-991 706 RNA copies/mL), whereas four (30.8%) were considered to be HIV-negative based on undetectable viral load results (<400 RNA copies/mL). Thus, a total of 13 results that had been positive according to the rapid test algorithm were actually negative. This yielded a false-positive rate of 0.6%.
Clinical records of cases with negative WB results were reviewed. After a minimum of 12 months with no clinical evidence of infection or laboratory evidence of HIV disease, these cases were discontinued from the clinic. The four cases with indeterminate WB results and undetectable viral load results were monitored for an additional two years. In the absence of any clinical manifestation of HIV infection and repeat undetectable viral load results, they were discharged from the clinic. Taking both the true positives and false positives into consideration, the positive predictive value was calculated to be 99.4% (99.0% - 99.6% CI). The satellite sites and private clinics were responsible for 46.2% (6/13) of the false-positive results. Upon further investigation, we found that all patients at these facilities (100%) had been screened using Determine and Stat-Pak test kits, including those with false-positive results. Since both kits produced concordant results, a third kit was not required as a tie breaker for any of these cases. The immunological and virological data of these false positives were analysed and it was found that their median CD4+ cell count was 668 cells/µL (range = 50-1500 cells/µL) and all had an undetectable viral load (< 400 RNA copies/mL).
Discussion
Rapid HIV antibody tests help to improve access to testing in both clinical and nonclinical settings, as well as increasing the proportion of people who receive their results once tested. As good as the rapid HIV screening assays may be, a confirmatory test is still required for a definitive diagnosis because of the possibility of false-positive results. False positives may result from numerous causes, including the inherent characteristics of the assay, or may be because of the genetic diversity of the virus.4 The percentage of false positivity reported in this study was 0.6%, which is similar to that reported in other studies,3,9 but is lower than the 2% reported in Cameroon.10 As part of Nigeria's global AIDS response progress report in 2012, 11.7% of the population of 162 265 000 had received an HIV test within the last 12 months and knew their HIV status.11 This corresponds to 18 985 005 tests performed in Nigeria within the year. Given the false-positive rate of 0.6% found in this study, approximately 113 910 people per year who are not HIV-positive receive a false HIV-positive diagnosis. The US President's Emergency Plan for AIDS Relief reported the yearly cost of management of an HIV patient to be US $338.12 These 113 910 false-positive diagnoses could result in US $38.5M per year in inappropriate but significant costs for HIV management. These resources could be better used for the estimated 1 512 720 HIV-infected individuals still requiring ART.
Possible ways to identify patients with false-positive results include: lack of risk factors for HIV, normal CD4+ cell count and undetectable viral load.13 In Nigeria, the CD4+ cell count reference value has been reported to be 365-1571 cells/µL.14 Causes of false-positive results include: fictitious HIV exposure/infection15 and HIV vaccination,16 the latter being unlikely because HIV vaccine trials have not been conducted in Nigeria. Other likely reasons for false-positive results could be either clerical or technical. The possibility of these errors in the NIMR virology reference laboratory is low because of the laboratory's stringent quality management system. None of the laboratories that carried out the HCT for the results included in this study had implemented a quality management system. This is not unusual, considering the dearth of knowledge about quality management systems in most laboratories in Africa, including Nigeria.17 The inconsistent or incorrect use of the rapid test algorithm has also been reported as a possible cause of false-positive results.18 In addition, false-positive results could also be a result of poor algorithm planning or implementation.19 The incorrect interpretation of weak-positive test lines and non-usage of tie-breaker algorithms, which occurs in our setting, could explain the latter.
The use of confirmatory WB allowed for the diagnosis of HIV-1 and HIV-2, between which these particular rapid test assays cannot discriminate. As seen in previous studies,20 HIV-1 was the major HIV type amongst patients accessing HIV care in our clinic. The very low rate of HIV-2 and HIV-1/2 dual infections observed in this study could be attributed to the decline in its prevalence, as reported in other West African countries,21 resulting in part from the inefficiency of transmission of HIV-2.22 In this study, men had a higher uptake of testing than women. This is similar to observations made between 2003 and 2007 (about the same timeframe as this study), but by 2011, this finding had been reversed.11 This is most likely attributable to the increased availability of prevention of mother-to-child transmission treatment, as well as treatment including paediatric therapy.
The delivery of a false-positive HIV result to a patient can be devastating, as it often results in psychological stress and trauma, particularly with subsequent stigmatisation. In a study from three African countries, namely, the Democratic Republic of Congo, Burundi and Ethiopia, some patients who were falsely diagnosed as HIV-positive had been abandoned by their partners after they started on ART or prophylaxis.23 There are also substantial financial costs related to misdiagnosis, where patients might lose time and compensation from work in order to attend clinic visits. In addition, the possible side effects and toxicities of unnecessary ART need to be considered. In our study, patients with false-positive results were found to have high median CD4+ cell counts and undetectable viral loads. Neither of these conditions can rule out the possibility of HIV infection, particularly if patients are natural elite controllers24 or receiving ART. It has also been reported that there are apparently healthy Nigerians who have CD4+ cell counts below 350 cells/µL.17,25
It is important to note that the cost of side effects, psychological trauma and lifelong treatment is unquantifiable. There have been reports of suicides resulting from a diagnosis of HIV,26 hence the gravity of false-positive results should be well-recognised. It is therefore essential that great care and caution is exercised in HIV diagnosis.
In spite of the challenge of false-positive results, the use of rapid test kits doubled the proportion of individuals who had been tested and received their test results between 2003 and 2007.11 The calculated positive predictive value of 99.4% in this study is similar to the established national positive predictive value of 99.5% (96.8% - 99.9% CI) based on the national algorithm's sensitivity of 100% and specificity of 99.7%.3 This implies that the use of this algorithm is still very essential in the expansion of HIV testing in a highly-populated country such as Nigeria, if the spread of this virus is to be controlled.
Limitations
This study is, however, not without limitations. The sensitivity, specificity and negative predictive values could not be estimated, because only HIV-positive patients were referred to the treatment centre for further follow up, including the WB re-testing. In like manner, not all cases referred from the HCT centres eventually reported to the treatment centre. It is also important to note that although WB is a confirmatory test, it does have a window period of six weeks; thus, its usefulness for the detection of acute infections is limited,27,28 as there is a possibility of missing early infections. Although rapid test kits also have a window period, the higher sensitivity assay is used initially, then followed by a very specific test. The long follow up of patients with discordant test results and repeat testing should have reduced the effect of the six-week window period. The WB assay also generates some indeterminate results. However, an indeterminate result is still acceptable in comparison to a false-positive result, as it allows for further evaluation.29
Conclusion
In this study, we found a 0.6% rate of HIV false positives with the implementation of the 2007 Nigerian HIV rapid testing algorithm. It is therefore recommended that clinicians should refer suspicious false-positive patients for WB confirmation. All possible measures should be taken to prevent false-positive diagnoses associated with rapid testing alone, as the long-term costs of treatment, side effects and psychological trauma to patients may be considerable.
Acknowledgements
We are grateful to the Harvard/AIDS Prevention Initiative in Nigeria (APIN) US President's Emergency Plan for AIDS Relief (PEPFAR) programme, which provided the financial support for this project. This work was supported, in part, by the US Department of Health and Human Services, Health Resources and Services Administration (U51HA02522). The contents are solely the responsibility of the authors and do not represent the official views of the funding institutions.
Competing interests
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article.
Authors' contributions
R.A.A (Nigerian Institute of Medical Research) was the project leader. R.N.O., C.K.O. and F.A.I. (Nigerian Institute of Medical Research) performed most of the experiments and retrieval of records. A.Z.M. (Nigerian Institute of Medical Research) was the data manager and responsible for data analysis. N.N.O., D.I.O. and O.C.E. (Nigerian Institute of Medical Research) were responsible for clinical management and manuscript preparation. E.O.I. (Nigerian Institute of Medical Research) made conceptual contributions, whilst P.J.K. (Harvard School of Public Health) was responsible for project design and manuscript preparation.
References
1. US Centers for Disease Control and Prevention, Advancing HIV prevention: new strategies for a changing epidemic - United States, 2003. MMWR. 2003;52(15):329-332. [ Links ]
2. UNAIDS/World Health Organization. Operational characteristics of commercially available assays to determine antibodies to HIV-1 and/or HIV-2 in human sera. Report 11. Geneva: World Health Organization; 1999. [ Links ]
3. Federal Ministry of Health, Nigeria. Laboratory based HIV rapid test validation in Nigeria, Phase 1, April 2007. Nigeria: The Nigeria HIV Rapid Test Evaluation Working Group; 2007. [ Links ]
4. Cordes RJ, Ryan ME. Pitfalls in HIV testing. Application and limitations of current tests. Postgrad Med. 1995;98(5):177-180, 185-186, 189. [ Links ]
5. Lyamuya EF, Aboud S, Urassa WK, et al. Evaluation of simple rapid HIV assays and development of national rapid HIV test algorithms in Dar es Salaam, Tanzania. BMC Infect Dis. 2009;9:19. http://dx.doi.org/10.1186/1471-2334-9-19 [ Links ]
6. Andersson S, da Silva Z, Norrgren H, et al. Field evaluation of alternative testing strategies for diagnosis and differentiation of HIV-1 and HIV-2 infections in an HIV-1 and HIV-2-prevalent area. AIDS. 1997;11(15):1815-1822. http://dx.doi.org/10.1097/00002030-199715000-00005 [ Links ]
7. Wesolowski LG, Delaney KP, Meyer WA, et al. Use of rapid HIV assays as supplemental tests in specimens with repeatedly reactive screening immunoassay results not confirmed by HIV-1 Western blot. J Clin Virol. 2013;58(1):240-244. http://dx.doi.org/10.1016/j.jcv.2013.06.019 [ Links ]
8. Baveewo S, Kamya MR, Mayanja-Kizza H, et al. Potential for false positive HIV test results with the serial rapid HIV testing algorithm. BMC Res Notes. 2012;5:154. http://dx.doi.org/10.1186/1756-0500-5-154 [ Links ]
9. Anzala O, Sanders EJ, Kamali A, et al. Sensitivity and specificity of HIV rapid tests used for research and voluntary counselling and testing. East Afr Med J. 2008; 85(10):500-504. [ Links ]
10. Aghokeng AF, Mpoudi-Ngole E, Dimodi H, et al. Inaccurate diagnosis of HIV-1 group M and O is a key challenge for ongoing universal access to antiretroviral treatment and HIV prevention in Cameroon. PLoS One. 2009;4(11):e7702. http://dx.doi.org/10.1371/journal.pone.0007702 [ Links ]
11. National Agency for the Control of AIDS, Federal Republic of Nigeria Global AIDS Response, Country Progress Report [document on the Internet]. c2012 [cited 2014 Sep 12]. [ Links ] Available from: http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/ 2012countries/ Nigeria2012GARPRReportRevised.pdf [updated link viewed 2015 Sep 07, from: http://www.unaids.org/sites/default/files/en/dataanalysis/knowyourresponse/ countryprogressreports/2012countries/ Nigeria%202012%20GARPR%20Report%20Revised.pdf]
12. Holmes CB, Atun R, Avila C, et al. Expanding the generation and use of economic and financial data to improve HIV program planning and efficiency: a global perspective. J Acquir Immune Defic Syndr. 2011;57(Suppl 2):S104-S108. http://dx.doi.org/10.1097/QAI.0b013e31821fa12d [ Links ]
13. Mylonakis E, Paliou M, Greenbough TC, et al. Report of a false-positive HIV test result and the potential use of additional tests in establishing HIV serostatus. Arch Intern Med. 2000;160 (15):2386-2388. http://dx.doi.org/10.1001/archinte.160.15.2386 [ Links ]
14. Oladepo DK, Idigbe EO, Audu RA, et al. Establishment of reference values of CD4 and CD8 lymphocyte subsets in healthy Nigerian adults. Clin Vaccine Immunol. 2009;16(9):1374-1377. http://dx.doi.org/10.1128/CVI.00378-08 [ Links ]
15. Craven DE, Steger KA, La Chapelle R, et al. Factitious HIV infection: the importance of documenting infection. Ann Intern Med. 1994;121(10):763-766. http://dx.doi.org/10.7326/0003-4819-121-10-199411150-00006 [ Links ]
16. Belshe RB, Clements ML, Keefer MC, et al. Interpreting HIV serodiagnostic test results in the 1990s: social risks of HIV vaccine studies in uninfected volunteers. NIAID AIDS Vaccine Clinical Trials Group. Ann Intern Med. 1994;121(8):584-589. http://dx.doi.org/10.7326/0003-4819-121-8-199410150-00005 [ Links ]
17. Gershy-Damet G, Rotz P, Cross D, et al. The World Health Organization African region laboratory accreditation process: improving the quality of laboratory systems in the African region. Am J Clin Pathol. 2010;134(3):393-400. http://dx.doi.org/10.1309/AJCPTUUC2V1WJQBM [ Links ]
18. Aniedobe MN, Onwuamah CK, Onubogu CC, et al. Proficiency of private laboratories in diagnosis of HIV in Nigeria. Oral Presentation at the 18th Union Conference, Africa Region, 3rd - 5th March 2011. Available from: http://www.afro.who.int/en/nigeria/press-materials/item/2884-the-18th-conference-of-the-international-union-against-tuberculosis-and-lung-disease-iuatld-african-region-held-from-3-5th-march-2011-abuja-nigeria.html [ Links ]
19. Klarkowski D, O'Brien DP, Shanks L, et al. Causes of false-positive HIV rapid diagnostic test results. Expert Rev Anti Infect Ther. 2014;12(1):49-62. http://dx.doi.org/10.1586/14787210.2014.866516 [ Links ]
20. Kashyap B, Gautam H, Chadha S, et al. Delayed progression and inefficient transmission of HIV-2. Southeast Asian J Trop Med Publ Health. 2010;41(3): 570-573. [ Links ]
21 Madec Y, Boufassa F, Porter K, et al. Spontaneous control of viral load and CD4 cell count progression among HIV-1 seroconverters. AIDS. 2005;19(17):2001-2007. http://dx.doi.org/10.1097/01.aids.0000194134.28135.cd [ Links ]
22. US Centers for Disease Control and Prevention. Interpretation and use of the Western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. MMWR. 1989;38(Suppl 7):1-7. [ Links ]
23. Owen SM, Yang C, Spira T, et al. Alternative algorithms for human immunodeficiency virus infection diagnosis using tests that are licensed in the United States. J Clin Microbiol. 2008;46(5):1588-1595. http://dx.doi.org/10.1128/JCM.02196-07 [ Links ]
24. Shanks L, Klarkowski D, O'Brien DP. False positive HIV diagnoses in resource limited settings: operational lessons learned for HIV programmes. PLoS One. 2013;8(3):e59906. http://dx.doi.org/10.1371/journal.pone.0059906 [ Links ]
25. Audu RA, Idigbe EO, Akanmu AS, et al. Values of CD4+ T lymphocyte in apparently healthy individuals in Lagos, Nigeria. EJSR. 2007;16(2):168-173. [ Links ]
26. Keiser O, Spoerri A, Brinkhof MW, et al.; Swiss HIV Cohort Study; Swiss National Cohort. Suicide in HIV-infected individuals and the general population in Switzerland, 1988-2008. Am J Psychiatry. 2010;167(2):143-150. http://dx.doi.org/10.1176/appi.ajp.2009.09050651 [ Links ]
27. Olaleye OD, Bernstein L, Ekweozor CC, et al. Prevalence of human immunodeficiency virus types 1 and 2 infections in Nigeria. J Infect Dis. 1993;167(3):710-714. http://dx.doi.org/10.1093/infdis/167.3.710 [ Links ]
28. Bock P, Markovitz D. Infection with HIV-2. AIDS. 2001;15(Suppl 5):S35-S45. http://dx.doi.org/10.1097/00002030-200100005-00006 [ Links ]
29. Ngan CCL, Thoe SYS, Chan KP, et al. Alternative strategies for confirmation of human immunodeficiency virus infection require judicious use. J. Clin. Microbiol. 2002;40(1):314-315. http://dx.doi.org/10.1128/JCM.40.1.314-315.2002 [ Links ]
 Correspondence:
Correspondence:
Rosemary Audu
Human Virology Laboratory, Nigerian Institute of Medical Research
06 Edmond Crescent, PMB 2013 Yaba
Lagos, Nigeria
Email: rosemaryaudu@yahoo.com
Received: 17 Mar. 2014
Accepted: 11 Aug. 2015
Published: 30 Sept. 2015














