Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.32 Pretoria 2024
http://dx.doi.org/10.17159/2254-8854/2024/a15718
RESEARCH ARTICLE
Biotic resistance towards Hydrellia egeriae, a biological control agent for the aquatic weed Egeria densa, in South Africa
Rosali MoffatI; Simon van NoortII, III; Julie CoetzeeI; Martin HillI
ICentre for Biological Control, Rhodes University, Makhanda, South Africa
IIResearch and Exhibitions Department, South African Museum, Iziko Museums of South Africa, Cape Town, South Africa
IIIDepartment of Biological Sciences, University of Cape Town, Rondebosch, South Africa
ABSTRACT
Egeria densa is a submerged aquatic weed that can grow into dense monocultures in rivers and dams in South Africa, which negatively affects ecosystem functioning and services. The biological control agent Hydrellia egeriae Rodrigues-Júnior (Diptera: Ephydridae) was first released against Egeria densa Planchon (Hydrocharitaceae) in South Africa in 2018. Biotic resistance in an introduced range can have negative impacts on the ability of a biological control agent to establish and exert top-down pressure. Dipteran and lepidopteran species that are used as biological control agents are often susceptible to higher levels of parasitism in their introduced range than biological control agents from other insect orders. In addition, ecological analogues that are present in South Africa, make H. egeriae particularly vulnerable to biotic resistance. Considering this, post-release surveys were conducted to investigate if native parasitoids will extend their host range to include H. egeriae. Chaenusa seminervata van Achterberg, C. anervata van Achterberg (Braconidae: Alysiinae: Dacnusini) and Ademon lagarosiphonae van Achterberg (Braconidae: Opiinae) were reared from field-collected H. egeriae pupae, within a year of its release. These braconid parasitoids were previously recorded from a native herbivore, Hydrellia lagarosiphon Deeming (Diptera: Ephydridae). Parasitism levels of H. egeriae ranged from 50 to 74% in cold months and 0 to 60% in warmer months, with higher levels of parasitism at a site where H. lagarosiphon naturally occurs. This study also found that cumulative release events of the biological control agent increase the probability of parasitism of field populations, by directly increasing the host pool. However, biological control efficacy can potentially be increased by limiting release efforts to a maximum of two release events per site per season, with particular focus on releasing in warm (i.e. spring/summer) months. Continued post-release surveys are necessary to not only monitor H. egeriae's impact on E. densa, but also to obtain a better understanding of seasonal parasitism levels across E. densa-invaded sites in South Africa.
Keywords: Braconidae; Ephydridae; parasitism; submerged aquatic weed; antagonism; Hydrocharitaceae.
INTRODUCTION
Following the successful management of invasive floating aquatic weeds in South Africa, empty niches have been filled by secondary invaders which include rooted emergent and submerged aquatic weeds (Coetzee et al. 2021). One of these secondary invaders is Egeria densa Planchon (Hydrocharitaceae) or Brazilian waterweed. It is a submerged aquatic weed that is native to South America, and it was introduced into South Africa in the late 19th or early 20th century (Cook and Urmi-König 1984). Only male plants occur in South Africa, and therefore its main mode of reproduction is vegetative growth.
In warm temperature conditions, E. densa can express different morphologies in response to seasonal changes; an herbaceous type with weak stems in summer and a robust grass type in winter (Haramoto and Ikusina 1988). Egeria densa does not have specialised storage organs (tubers and turions), but stores carbohydrates in its leaves, stems, and roots to survive colder temperatures (Haramoto and Ikusina 1988). In tropical and subtropical regions, E. densa does not exhibit bimodal biomass patterns but grows actively year-round with its highest biomass in summer (Mazzeo et al. 2003).
Egeria densa grows vigorously in polluted or degraded freshwater systems, which allows it to form dense monocultures that crowd out native fauna and flora and restrict water usage. Any plant fragment with a double node has the potential to develop into a new individual. Considering this, and its ability to easily spread to new sites in South Africa, Hydrellia egeriae Rodrigues-Júnior (Diptera: Ephydridae), native to Argentina, was imported via the U.S.A., and released as a biological control agent in 2018 after a risk assessment confirmed that it is safe for release (Smith et al. 2019).
Introduced organisms can reach high population levels in their novel environment, because they are released, partially or completely, from their co-evolved enemies (Keane and Crawley 2002; Heimpel and Mills 2017; Schulz et al. 2019). However, previous work has shown that weed and insect biological control agents often experience antagonism or biotic resistance in their introduced range (Cornell and Hawkins 1993; Hill and Hulley 1995; Paynter et al. 2010; Hill and Coetzee 2020), which reduces their capacity to exert top-down control on their host species. The number of parasitoids that introduced species acquire in their introduced range (Kelly et al. 2009) is often a reflection of the number of parasitoids (species from the orders Hymenoptera and Diptera) they have in their native range (Cornell and Hawkins 1993; Paynter et al. 2020). Parasitoids of weed biological control agents in the introduced range are typically generalists (Cornell and Hawkins 1993), but specialist parasitoids can also extend their host range if the biological control agent is closely related to a native host (Godfray et al. 1995). The feeding guild of the biological control agent has also been identified as a predictor of potential parasitism prior to release. Hill and Hulley (1995) found that in South Africa, poorly concealed endophagous agents were the most affected with 62.5% of introduced species in this guild being parasitised, followed by well-concealed endophages (47.1%) and ectophages (20%). When biological control agents were grouped into insect orders, Hill and Hulley (1995) also concluded that insect order may be a predictor of parasitism. Biological control agents within the orders Diptera and Lepidoptera are most attacked, while other orders are less likely to acquire parasitoids.
The shorefly family Ephydridae has approximately 2 000 described species that are distributed worldwide. Species within the family are generally herbivores of aquatic and semi-aquatic plants. Natural enemies of the genus Hydrellia are well documented (Deonier 1971; Stiling et al. 1984; Kula et al. 2009; Cabrera Walsh et al. 2012; Coon et al. 2014; Katzenberger and Zacharias 2015), and the most common parasitoid family associated with Hydrellia spp. is Braconidae (Goulet and Huber 1995). In its native range, H. egeriae parasitism levels were 10.4% and included the parasitoids Chaenusa aurantium Kula and Martinez (Hymenoptera: Braconidae), Hydrelliaeucoila egeria Díaz and Gallardo (Hymenoptera: Figitidae), and an unidentified pupal fungus (Cabrera Walsh et al. 2012). These parasitoid species do not occur in South Africa, but other Hydrellia spp. parasitoids occur in South Africa, with higher parasitism levels reported in cold months compared to warmer months (Martin et al. 2013). Considering the phylogenetic relatedness of both host-parasitoid associations (Paynter et al. 2010), we hypothesise that H. egeriae will experience parasitism in its introduced range in South Africa. We also hypothesise that similar to H. egeriae's ecological analogue, parasitism levels will be higher in cold months when the insect enters diapause.
MATERIALS AND METHODS
Site descriptions
The presence of parasitoids and the percentage of H. egeriae's population that was parasitised, were measured quarterly at two sites between May 2019 and September 2020. The first study site was in the Nahoon River (-32.962777; 27.911388) in the Eastern Cape province. The site is situated approximately 22.7 km downstream from the Nahoon Dam (est. 1966), and approximately 500 m before a weir that leads the freshwater river into an estuary. The river is approximately 55 m wide at the site and holds the highest density of E. densa in the Nahoon River. The second site is Midmar Dam (-29.531156; 30.203999) located in the KwaZulu-Natal province. The dam's surface area is approximately 1 880 ha, and E. densa distribution is mostly localised in the bays of the dam. A subset of the dam was selected for the study, mainly because of easy accessibility and limited time to achieve study aims.
Study organism
Hydrellia egeriae is a multivoltine leaf-mining fly. Adults are 1.3-3.0 mm in length and naturally feed on nectar, fungi, and dead insects. Females oviposit on protruding E. densa leaves or other available substrates (Smith et al. 2019). Upon eclosion, the first larval instar feeds in the mesophyll layer of an E. densa leaf. Larvae create entry scars in the epidermal layer of a leaf with their mouth hooks. First instars usually feed in the crown of the shoot, where leaves are softer. Older instars feed on leaves throughout the plant. Hydrellia egeriae has three instars, before pupariation at the base of the last leaf it mined. Upon emergence, adults float to the water surface in an air bubble (Cabrera Walsh et al. 2013; Smith et al. 2019). Egg to adult development at a constant 25 °C takes a month to complete (Cabrera Walsh et al. 2013), and previous work has shown that H. egeriae's thermal physiology is wider than that of its host plant (Smith et al. 2022).
Release events
Only one release of H. egeriae was made at the Nahoon River (October 2018), while four releases were made at Midmar Dam (March 2019 - March 2020). Hydrellia egeriae was mass-reared in large portable pools in a polytunnel at the Waainek Research Facility, Rhodes University, Makhanda, South Africa, allowing the insects to develop under semi-natural conditions. A population count of H. egeriae at the mass-rearing facility was done every month or two months and the population size was calculated as the number of immatures (eggs, larvae, pupae) per kilogram fresh weight of E. densa.
For release events, the largest number of available H. egeriae was collected from the mass-rearing population. Hydrellia egeriae was released by transporting E. densa containing immatures from the mass-rearing facility to the release site and placing it in the water. To estimate the number of biological control agents released for each release event, the corresponding population size for that month (immatures/kg fresh weight E. densa) and the weight of collected plant material was used. The release effort for this study is illustrated in Table 1. The total number of H. egeriae immatures released at Midmar Dam was 59 926, and 10 071 for the Nahoon River.
Parasitoid collection
Egeria densa plant material containing H. egeriae individuals was collected from the two field sites in order to rear out potential parasitoids. This was done in conjunction with H. egeriae post-release surveys. Five collections were made from the Nahoon River (May 2019, July 2019, December 2019, July 2020, September 2020), and four collections were made from Midmar Dam (June 2019, October 2019, March 2020, August 2020).
Egeria densa was collected at each site along a pre-determined, fixed transect. In the Nahoon River, the transect was parallel with the riverbed and spanned 58 m in length. The transect in Midmar Dam consisted of two shorter transects parallel to the shoreline. Shorter transects were selected because a single, long transect would traverse areas in the dam that were too deep to wade through. Taken together, the transect was approximately 167 m in length.
A quadrat (0.5 m χ 0.5 m) was randomly thrown at the start of a transect. Three to five quadrats (0.5 m χ 0.5 m in size) of E. densa were collected at each site. All the E. densa found within a quadrat (< 1 m in water depth) was collected by hand and placed in a black plastic bag. Water was drained and bags were placed in cooler boxes containing ice packs to keep conditions cool during transportation. In the case of Midmar Dam only three quadrats were collected because plant material was transported by aeroplane back to the laboratory. Five quadrats were collected from the Nahoon River, because plant material was transported by car back to the laboratory. At the laboratory, the bags were placed in a 5 °C fridge until dissected. Every E. densa shoot (20 to 30 cm), in a sample bag was inspected for H. egeriae pupae. The pupal life stage was selected because previous studies have shown that Hydrellia spp. parasitoids generally feed on late-instar larvae and on pupae (Deonier 1971; Kula et al. 2009; Martin et al. 2013; Coon et al. 2014). Once a puparium was found, the E. densa fragment containing the pupa was broken off with forceps and placed in a vial containing fresh water. The number of pupae collected from all quadrats per sampling event at each of the two sites was pooled. Vials were placed in a laboratory at room temperature and checked every second day for H. egeriae adult emergence or parasitoid emergence. This provided the number of parasitoids that emerged from the field-collected H. egeriae pupae per sampling event.
Eclosed insects were collected and placed in an Eppendorff™ containing 96% ethanol. Parasitoid specimens were identified by one of the authors (SvN; van Noort et al 2021) and voucher specimens were deposited at SAMC: Iziko South African Museum, Cape Town.
Statistical analysis
Percentage parasitism
The proportion of parasitism for each sampling event was calculated by dividing the number of emerged parasitoids by the total number of H. egeriae pupae collected. This proportion was used to calculate the percentage parasitism for each sampling event. To determine if parasitism was different between seasons, sampling events, and thus parasitism levels, were grouped into cold months (March-August) and warm months (September-February). Only two groups instead of Spring, Summer, Autumn, Winter were used due to limited sampling events.
To determine if sampling period and site had a significant effect on H. egeriae parasitism, a generalised linear model (GLM) was used. Hydrellia egeriae parasitism (yes/no) was modelled as a linear function of sampling period (cold and warm), assuming a binomial error distribution with a logit link function. The DHARMa package (Hartig 2022) was used to plot a graph of the model and check for violation of homogeneity. Thereafter, the statistical significance of the model was assessed by performing a likelihood ratio test (p < 0.05). The Tukey post-hoc analysis test was performed to test for statistical differences (p < 0.05) between sites within the period treatment.
Effect of cumulative release events on parasitism
To investigate the probability of H. egeriae parasitism in the field as a function of cumulative release events, data from Midmar Dam and Nahoon River were combined. Hydrellia egeriae parasitism (yes/no) was modelled as a function of the number of H. egeriae releases using a generalised linear model (GLM), assuming a binomial error distribution with a logit link function. The DHARMa package (Hartig 2022) was used to plot a graph of the model and check for violation of homogeneity. Thereafter, the statistical significance of release events on the probability of H. egeriae parasitism in the field was assessed by performing a likelihood ratio test (p < 0.05). All analyses and graphs were done in the R environment (R Core Team 2022).
RESULTS
Hydrellia egeriae was parasitised by three species of native parasitoids: Chaenusa seminervata van Achterberg, C. anervata van Achterberg (Braconidae: Alysiinae: Dacnusini), and Ademon lagarosiphonae van Achterberg (Braconidae: Opiinae) (van Noort et al 2021). These species were previously recorded from Hydrellia lagarosiphon Deeming (Diptera: Ephydridae) a specialist herbivore of Lagarosiphon major (Ridl.) Moss ex Wager (Hydrocharitaceae) in South Africa (van Achterberg & Prinsloo 2012).
Percentage parasitism
The percentage parasitism for the study period ranged from 20.0 to 87.4% in Midmar Dam and 0.0 to 84.3% in the Nahoon River. Hydrellia egeriae parasitism levels in Midmar Dam were 74.32 ± 3.23% (n = 3) in cold months and 60.0 ± 16.33% (n = 1) during warm months. In the Nahoon River, parasitism levels were 50.0 ± 4.44% (n = 3) in cold months with no (n = 2) parasitism occurring in warm months (Figure 1).
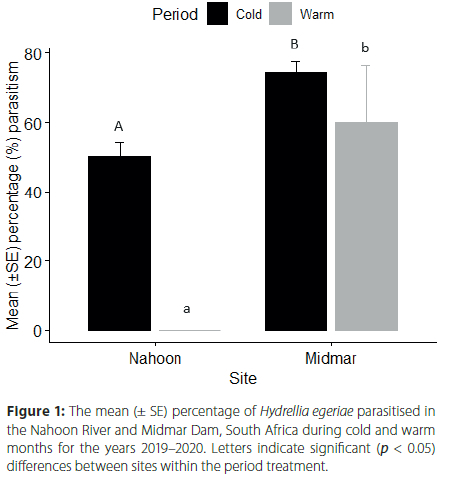
The interaction of site and sampling period on H. egeriae parasitism was significant (χ2 = 5.55, df = 1, p < 0.05). Parasitism of H. egeriae was also significantly affected by site (χ2 = 24.77, df = 1, p < 0.001) and sampling period (χ2 = 8.50, df = 1, p < 0.01) individually. Tukey post-hoc analysis showed that H. egeriae parasitism was significantly higher in the Midmar Dam during both cold (p < 0.001) and warm months (p < 0.001) than in the Nahoon River (Figure 1).
Effect of cumulative release events on parasitism
The cumulative number of H. egeriae release events significantly increased the probability of agent parasitism in the field (β1 = 1.93, χ2 = 55.83, df = 1, p < 0.001). The model shows that for every additional release event, the probability of parasitism increased (Figure 2). After two release events the probability of parasitism in the field was approximately 60%, and after eight release events the probability of parasitism, nearly 100%.
DISCUSSION
Parasitoids that attacked H. egeriae in South Africa were previously recorded from H. lagarosiphon (van Achterberg and Prinsloo 2012), a specialist herbivore of L. major in South Africa (van Noort et al. 2021). These parasitoids have thus extended their host range to H. egeriae within a year of releasing this biological control agent. This is not surprising due to the phylogenetic relatedness of the two herbivore species and their host plants (McFadyen and Spafford Jacob 2003; Paynter et al. 2010). Martin et al. (2013) found that the native herbivore, H. lagarosiphon, had parasitism rates that ranged from 13 to 28% in summer and 0 to 52% in winter. Similarly, in our study, H. egeriae parasitism was higher in the cold months (50 to 74%) than in the warm months. The higher parasitism rates in winter could be linked to the apparency of the host plant throughout the year, meaning the host plants are perennial and available to their herbivores throughout the year (McFadyen and Spafford Jacob 2003). Both Hydrellia species are multivoltine, and the temperate and subtropical climates may favour population buildup throughout the year (McFadyen and Spafford Jacob 2003). During cold months, H. lagarosiphon and H. egeriae enter diapause, and overwinter mostly in the immobile pupal stage. Hydrellia spp. parasitoids generally prefer late-instar larvae or pupae (Deonier 1971; Kula et al. 2009; Martin et al. 2013; Coon et al. 2014), and thus, parasitoids could benefit from a large pool of vulnerable hosts during cold months.
All three parasitoids, C. seminervata, C. anervata and A. lagarosiphonae, were collected from Midmar Dam, while only A. lagarosiphonae was collected from the Nahoon River. These collections are within the known distribution range of the parasitoids. Chaenusa seminervata and C. anervata occur in the Mpumalanga and KwaZulu-Natal provinces, while A. lagarosiphon occurs in the Mpumalanga, KwaZulu-Natal, and Eastern Cape provinces (van Achterberg and Prinsloo 2012). It is plausible that the proximity ofnative host plants of H. lagarosiphon could have contributed to higher parasitoid richness in Midmar Dam compared to the Nahoon River. No Lagarosiphon species occur in the Nahoon River, while Lagarosiphon. muscoides Harv. (Hydrocharitaceae) and Potamogeton species (Potamogetonaceae) occur in the Midmar Dam. The leaf-mining fly H. lagarosiphon has been collected from L. muscoides and Potamogeton species (Mangan et al. 2019). However, differences in parasitoid richness between the two sites could also be an artefact of differences in release efforts, and sampling size.
The high levels of parasitism recorded in this study will affect field populations of H. egeriae, and thus inundative releases will be necessary to achieve better control of E. densa. However, inundative releases may indirectly increase the probability of H. egeriae parasitism by increasing the host pool. Therefore, to contribute to the biological control of E. densa in South Africa, while minimising parasitism in the field, it is recommended that release events take place during the warm (i.e. spring/summer) months. Inundative releases should commence after winter to assist low field populations, but release events should be limited to a maximum of two releases per site per season.
Parasitism of biological control agents in their introduced range has received some attention over the decades, with the first review on this topic published by Goeden and Louda (1976). With the accumulation of information, guidelines were given on the selection of biological control agents with particular focus on avoiding parasitism in the target country (McFadyen and Spafford Jacob 2003; Paynter et al 2010). This includes the phylogenetic relatedness in the country of introduction and the feeding niche and taxonomy of the insect etc. (McFadyen and Spafford Jacob 2003; Paynter et al 2010). For example, Cornell and Hawkins (1993) found the lowest (2.2%) parasitoid richness on herbivores with a mixed feeding niche and the highest values (7.7%) on herbivores that are endophytic and poorly concealed. This means that introduced herbivores with larval stages that are both exophytic and endophytic may acquire fewer parasitoids, compared to herbivores that are conspicuous, as in the case of H. egeriae. Parasitoid richness may also be a measure of host mortalities, as seen in a meta-analysis of 2 188 holometabolous species from 86 countries by Hawkins (1993). In this study, herbivores (i.e., case bearers, leaf-miners, gallers) with high parasitoid richness experienced higher mortality rates compared to herbivores (i.e., root feeders, borers, mixed) with low parasitoid richness.
Biotic resistance towards introduced biological control agents has led to low-impact or failed biological control programmes, for example the gall fly, Procecidochares utilis Stone (Diptera: Tephritidae), a biological control agent released against Ageratina adenophora (Spreng.) King and H. Rob. (Asteraceae) in Thailand, Australia, India, New Zealand, Hawaii, China, Nepal, and South Africa. In Hawaii, the dipteran agent acquired four parasitoids, and surveys between 1950 and 1957, and 1966 and 1971, showed that parasitism rates ranged from 30 to 93% in winter and 7 to 72% in summer (Bess and Haramoto 1972). The efficacy of the agent in Hawaii varies, depending on the local climate, parasitism, and predation (Winston et al. 2023).
Some biological control programmes only have a limited list of prospective agents to choose from, as in the case of E. densa (Cabrera Walsh et al. 2013). In such cases, biological control agents should not be disregarded due to possible parasitism in their introduced range (Hill and Hulley 1995; van Klinken and Burwell 2005). Parasitism of biological control agents is undesirable, not only because of its impact on the efficacy of the biological control programme, but also because of its indirect effect on native food webs (Paynter et al 2020). Hydrellia species are poorly studied in South Africa, and the parasitoids recorded in this study were initially discovered due to a classical biological control programme on L. major (Martin et al. 2013; van Achterberg and Prinsloo 2012). It is highly likely that a larger parasitoid host pool, which would occur if H. egeriae populations remained high, could increase parasitism pressure on native hosts, especially if there is synchrony between host species. However, a potential hyperparasitoid, Janicharis africanus Gumovsky and Delvare (Hymenoptera: Eulophidae) has also been reared from field-collected H. egeriae (van Noort et al. 2021). Thus, the parasitoids recorded and monitored in this study are likely experiencing top-down pressure. Alterations to food web interactions are difficult to establish, but continued post-release surveys can assist in mapping the seasonal abundance and distribution of parasitoids and hyperparasitoids at E. densa-invaded sites in South Africa.
ACKNOWLEDGMENTS
This research was funded through the South African Department of Forestry, Fisheries and the Environment, Natural Resource Management Programmes. Further funding for this work was provided by the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation of South Africa (grant numbers 89967, 109244 and 109683 to Martin Hill, SARCHi Chair). Simon van Noort was funded by a South African NRF FBIP grant number 98115. Thanks are extended to the editors and anonymous reviewers for their valuable comments, which has improved the quality of this manuscript.
AUTHOR CONTRIBUTIONS
RM: conceptualisation, methodology, formal analysis, investigation, writing, visualisation; SvN: methodology, investigation, writing;
JC: conceptualisation, writing, supervision, project administration, funding acquisition;
MH: conceptualisation, writing, supervision, project administration, funding acquisition.
ORCID IDS
Rosali Moffat: https://orcid.org/0000-0003-3010-7493
Simon van Noort: https://orcid.org/0000-0001-6930-9741
Julie Coetzee: https://orcid.org/0000-0002-0364-3349
Martin Hill: https://orcid.org/0000-0003-0579-5298
REFERENCES
Bess HA, Haramoto FH. 1959. Biological control of Pamakani, Eupatorium adenophorum, in Hawaii by a tephritid gall fly, Procecidochares utilis. 2. Population studies of the weed, the fly, and the parasites of the fly. Ecology. 40(2):244-249. https://doi.org/10.2307/1930034. [ Links ]
Cabrera Walsh G, Magalí Dalto Y, Mattioli FM, Carruthers RI, Anderson LW. 2013. Biology and ecology of Brazilian elodea (Egeria densa) and its specific herbivore, Hydrellia sp., in Argentina. BioControl 58: 133-147. https://doi.org/10.1007/s10526-012-9475-x. [ Links ]
Coetzee JA, Bownes A, Martin GD, Miller BE, Smith R, Weyl PSR, Hill MP. 2021. A review of the biocontrol programmes against aquatic weeds in South Africa. African Entomology 29(3): 935-964. https://doi.org/10.4001/003.029.0935. [ Links ]
Cook CDK, Urmi-König K. 1984. A revision of the genus Egeria (Hydrocharitaceae). Aquatic Botany 19: 73-96. https://doi.org/10.1016/0304-3770(84)90009-3. [ Links ]
Coon BR, Harms NE, Cuda JP, Grodowitz MJ. 2014. Laboratory biology and field population dynamics of Trichopria columbiana (Hymenoptera: Diapriidae), an acquired parasitoid of two hydrilla biological control agents. Biocontrol Science and Technology 24(11): 1243-1264. https://doi.org/10.1080/09583157.2014.933311. [ Links ]
Cornell HV, Hawkins BA. 1993. Accumulation of native parasitoid species on introduced herbivores: A comparison of hosts as natives and hosts as invaders. The American Naturalist 141(6): 847-865. https://doi.org/10.1086/285512. [ Links ]
Deonier DL. 1971. A systematic and ecological study of Nearctic Hydrellia (Diptera: Ephydridae). Smithsonian Contributions to Zoology 68: 1-147. https://doi.org/10.5479/si.00810282.68. [ Links ]
Godfray CJ, Agassiz DJL, Nash DR, Lawton J. 1995. The recruitment of parasitoid species to two invading herbivores. Journal of Animal Ecology 64: 393-402. https://doi.org/10.2307/5899. [ Links ]
Goeden RD, Louda SM. 1976. Biotic interference with insects imported for weed control. Annual Review of Entomology 21: 325-342. https://doi.org/10.1146/annurev.en.21.010176.001545. [ Links ]
Goulet H, Huber JT. 1995. Hymenoptera of the world: an identification guide to families. Research Branch, Agriculture Canada. Ottawa, Ontario. 680 pp. https://doi.org/10.1002/mmnd.19950420212.
Haramoto T, Ikusima I. 1988. Life cycle of Egeria densa Planch., an aquatic plant naturalized in Japan. Aquatic Botany 30: 389-403. https://doi.org/10.1016/0304-3770(88)90070-8. [ Links ]
Hartig F. 2022. DHARMa: Residual diagnostics for hierarchical (Multi-Level / Mixed) Regression Models. R package version 0.4.6. https://CRAN.R-project.org/package=DHARMa.
Hawkins BA. 1993. Species richness, host mortality, and biological control. The American Naturalist 141(4): 634-641. https://doi.org/10.1086/285495. [ Links ]
Heimpel GE, Mills N. 2017. Biological control: Ecology and Applications. Cambridge: Cambridge University Press. 386 pp. https://doi.org/10.1017/9781139029117. [ Links ]
Hill MP, Hulley PE. 1995. Host range extension by native parasitoids to weed biocontrol agents introduced to South Africa. Biological Control 5: 297-302. https://doi.org/10.1006/bcon.1995.1037. [ Links ]
Hill MP, Coetzee JA. 2020. Chapter 20. How can progress in the understanding of antagonistic interactions be applied to improve biological control of plant invasions? In: Traveset A, Richardson DM, editors. Plant Invasions: The Role of Biotic Interactions. Wallingford: CABI. pp 363-374. http://dx.doi.org/10.1079/9781789242171.0020. [ Links ]
Katzenberger J, Zacharias D. 2015. Mutualism of Stratiotes aloides L. (Hydrocharitaceae) and Hydrellia tarsata Haliday (Diptera: Ephydridae): tritrophic interaction of macrophyte, leaf-mining dipteran pollinator and parasitoid Braconidae. Journal of Pollination Ecology 15(4): 23-29. https://doi.org/10.26786/1920-7603(2015)3. [ Links ]
Keane RM, Crawley MJ. 2002. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology and Evolution. 17(4): 164-170. http://dx.doi.org/10.1016/S0169-5347(02)02499-0. [ Links ]
Kelly DW, Paterson RA, Townsend CR, Poulin R, Tompkins DM. 2009. Parasite spillback: A neglected concept in invasion ecology? Ecology 90(8): 2047-2056. http://dx.doi.org/10.1890/08-1085.1. [ Links ]
Kula RR, Martinez JJ, Cabrera Walsh G. 2009. Supplement to revision of New World Chaenusa Haliday SensuLato (Hymenoptera: Braconidae: Alysiinae). Proceedings of the Entomological Society of Washington 111(3): 641-655. https://doi.org/10.4289/0013-8797-11L3.641. [ Links ]
Mangan R, Carolan JC, Baars JR. 2019. Molecular characterization of Hydrellia lagarosiphon, a leaf-mining biological control agent for Lagarosiphon major, reveals weak variance across large geographic areas in South Africa. Biological Control. 132: 8-15. http://dx.doi.org/10.1016/j.biocontrol.2019.01.017. [ Links ]
Martin GD, Coetzee JA, Baars JR. 2013. Hydrellia lagarosiphon Deeming (Diptera: Ephydridae), a potential biological control agent for the submerged aquatic weed, Lagarosiphon major (Ridley) Moss (Hydrocharitaceae). African Entomology 21(1): 151-160. http://dx.doi.org/10.4001/003.021.0118. [ Links ]
Mazzeo N, Rodríguez-Gallego L, Kruk C, Meerhoff M, Gorga J, Lacerot G, Quintans F, Loureiro M, Larrea D, García-Rodríguez F. 2003. Effects of Egeria densa Planch. beds on a shallow lake without piscivorous fish. Hydrobiologia 506-509(1-3): 591-602. [ Links ]
McFadyen R, Spafford Jacob H. 2003. Insects for the biocontrol of weeds: predicting parasitism levels in the new country. In: Cullen JM, Briese DT, Kriticos DJ, Lonsdale WM, Morin L, Scott JK. (Eds.) Proceedings of the XI International Symposium on Biological Control of Weeds. Canberra: CSIRO Entomology. pp. 135-140. [ Links ]
Paynter Q, Fowler SV, Hugh-Gourlay A, Groenteman R, Peterson PG, Smith L, Winks CJ. 2010. Predicting parasitoid accumulation on biological control agents of weeds. Journal of Applied Ecology 47: 575-582. https://doi.org/10.1111/j.1365-2664.2010.01810.x. [ Links ]
Paynter Q, Paterson ID, Kwong RM. 2020. Predicting nontarget impacts. Current Opinion in Insect Science. 38: 79-83. https://doi.org/10.1016/jxois.2020.02.002. [ Links ]
R Core Team. 2022. R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. https://www.R-project.org/.
Schulz AN, Lucardi RD, Marsico TD. 2019. Successful invasion and failed biocontrol: The role of antagonistic species interactions. BioScience 69(9): 711-724. https://doi.org/10.1093/biosci/biz075. [ Links ]
Smith R, Coetzee JA, Hill MP. 2022. Best of both worlds: the thermal physiology of Hydrellia egeriae, a biological control agent for the submerged aquatic weed, Egeria densa in South Africa. BioControl 67(3): 365-374. https://doi.org/10.1007/s10526-022-10142-w. [ Links ]
Smith R, Mangan R, Coetzee JA. 2019. Risk assessment to interpret the physiological host range of Hydrellia egeriae, a biocontrol agent for Egeria densa. BioControl. 64: 447-456. http://dx.doi.org/10.1007/s10526-019-09942-4. [ Links ]
Stiling PD, Brodbeck BV, Strong DR. 1984. Intraspecific competition in Hydrellia valida (Diptera: Ephydridae), a leaf-miner of Spartina alterniflora. Ecology. 65(2): 660-662. https://doi.org/10.2307/1941431. [ Links ]
van Achterberg C, Prinsloo GL. 2012. Braconidae (Hymenoptera: Opiinae, Alysiinae) reared from aquatic leaf-mining Diptera on Lagarosiphon major (Hydrocharitaceae) in South Africa. African Entomology. 20(1): 124-133. https://hdl.handle.net/10520/EJC119297. [ Links ]
van Klinken RD, Burwell CJ. 2005. Evidence from a gelechiid leaf-tier on mesquite (Mimosaceae: Prosopis) that semi-concealed lepidopteran biological control agents may not be at risk from parasitism in Australian rangelands. Biological Control 32(1): 121-129. https://doi.org/10.1016/j.biocontrol.2004.09.001. [ Links ]
van Noort S, Smith R, Coetzee JA. 2021. Identity of parasitoid wasps (Hymenoptera: Braconidae and Eulophidae) reared from aquatic leaf-mining flies (Diptera: Ephydridae) on invasive Brazilian waterweed Egeria densa in South Africa. African Invertebrates 62(1): 287-314. https://doi.org/10.3897/afrinvertebr.62.62842. [ Links ]
Winston RL, Schwarzlander M, Hinz HL, Day MD, Cock MJW, Julien MH, Eds. 2023. Biological Control of Weeds: A World Catalogue of Agents and Their Target Weeds. Based on FHTET-2014-04, USDA Forest Service, Forest Health Technology Enterprise Team. Available online at https://www.ibiocontrol.org/catalog/ [Accessed 07 December 2023].
 Correspondence:
Correspondence:
Rosali Moffat
Email rosali.moffat@fabi.up.ac.za
Received: 3 March 2023
Accepted: 16 February 2024














