Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a16150
RESEARCH ARTICLE
In vitro liquid mass production of a South African isolate of Heterorhabditis zealandica
Murray D. Dunn; Antoinette P. Malan
Department of Conservation Ecology and Entomology, Faculty of AgriSciences, Stellenbosch University, Stellenbosch, South Africa
ABSTRACT
Developing repeatable protocols for the in vitro liquid mass production of entomopathogenic nematodes (EPNs) is a difficult task and depends on the nematode species being cultured. Of critical importance is the establishment of a monoxenic population of nematodes as a stock culture for optimisation and experimental purposes. Establishing a new stock inoculum culture flask of pure infective juveniles (IJs) is challenging, particularly for the Heterorhabditis species, due to their affinity for developing into an amphimictic second generation that does not copulate in liquid culture flasks. Developing mass production protocols for multiple EPNs is advisable because different pest insects are susceptible to different species of EPN. This study attempted to mass-produce a South African isolate of Heterorhabditis zealandica and its symbiotic bacteria, Photorhabdus thracensis, using in vitro liquid culture technology methods previously developed for Steinernema species. The results indicate that the pre-culture protocols developed for Steinernema species are applicable to a H. zealandica isolate. Moreover, the results, in terms of the protein source optimisation experiments, confirm that different EPN species have different culture conditions and nutrient requirements, with H. zealandica seeming to prefer soy-based protein instead of egg yolk, having higher recovery and producing more hermaphrodites, using soy protein. This study illustrates the importance of developing dependable and infallible preculture methods, prior to the flask mass production process.
Keywords: biocontrol agents, egg yolk, entomopathogenic nematodes, Photorhabdus thracensis, soy
INTRODUCTION
The in vitro liquid mass production of entomopathogenic nematodes (EPNs) is an advanced biotechnological process (Ehlers 2001), spanning multiple scientific disciplines, including microbiology, nematology, process engineering and biochemistry. Over the last two decades, the amount of literature concerning this process has grown immensely, allowing new research teams to rapidly develop suitable methods and techniques for the mass production of their own local isolates. However, the initial stages of mass production, such as creating monoxenic cultures of nematodes and providing the growing nematodes with a suitable diet, are still challenging (Strauch and Ehlers 1998; Yoo et al. 2000; Ehlers 2001). Additionally, although the amount of literature concerning flask-level production has grown, the same cannot be said of the large-scale bioreactor research, which is still scarce in comparison (Dunn et al. 2021). Thus, developing a technique of mass production for new EPN species takes time, and requires a multidisciplinary team effort.
An additional issue is that, when research teams develop their methods and protocols for EPN mass production, the methods are not always completely monoxenic, due to the method of nematode sterilisation used. The two most common methods of nematode sterilisation are the IJ surface sterilisation technique, and the egg sterilisation technique (Ehlers 2001; Dunn et al. 2021), with the latter being far more dependable in creating a truly monoxenic environment. New species often require vastly different methods to mass-produce, compared to other species. Thus, many of the protocols that have been developed seem to have limited yields and long production times, because they are either contaminated or have insufficient nutrient composition in the nutrient medium, which is exacerbated when trying to culture multiple species.
Developing mass production protocols and techniques for multiple species is important, as it is unwise to rely on a single isolate for insect control, in other words, "do not put all your eggs in one basket". Such is often the case with new companies, as they tend to develop a method for a particular species and to market it as a high-performing, wide-range biopesticide. While such might, to some degree, be true, having the option of a second, or even a third product, with different EPN species or isolates is far more reliable, as different species are likely to be better suited to specific insects and environmental conditions (Lewis et al. 1992; Grewal et al. 1994; Hominick 2002; Torr et al. 2007), and ensures that growers do not lose faith in an EPN product when it does not work as was originally intended (Warrior 2000). Thus, it is advisable to develop mass production techniques for more than one EPN species.
An important factor is the genus of the EPN being produced. EPNs of the genera Heterorhabditis and Steinernema and their associated symbiotic bacteria, Photorhabdus and Xenorhabdus, respectively (Boemare et al. 1993), are the two most common genera of EPN, with every major commercial-producing company worldwide producing either of the genera (Kaya et al. 2006). The two genera do not belong to the same family, which has led to key differences in their biology, although their lifestyle is the same. The two main differences, which are specifically important for mass culture, between the two genera are their association with their genus of bacterial symbiont, and their method of reproduction with Heterorhabditis having only hermaphrodites (self-fertilising females) in the first generation and being amphimictic in the second (Poinar 1975, 1990), with both females and males, while Steinernema is always amphimictic in the first and all following generations (Strauch et al. 1994).
The bacterial symbionts, Photorhabdus and Xenorhabdus, possess biochemical traits that assist the nematode in overcoming the host's immune response, breaking down the inner body of the host for the developing nematodes to consume, and protecting the cadaver from unwanted microbes and other predatory insects (Peters and Ehlers 1994). Though the two bacterial genera have biochemical traits that perform similar functions, major differences exist between them (Forst and Nealson 1996), with such differences becoming extremely important for the in vitro liquid mass production process.
The mode of reproduction is, similarly, an important factor, as multiple variables that impact the in vitro liquid process of Steinernema often do not apply to Heterorhabditis. The most subtle factor is that Steinernema requires two nematodes to produce offspring, whereas Heterorhabditis only requires one nematode, as its first-generation adult stage is hermaphrodite (Poinar 1975, 1990). Thus, in the case of Heterorhabditis, no need exists for contact between two adults of different sexes in the flask environment, as is the case for Steinernema. It is advantageous to have a high inoculum concentration for Steinernema when recovery (the process whereby IJs exit the dormant IJ stage and initiate the life cycle in the presence of a favourable reproductive environment) is highly variable, because a higher recovery rate for a larger population means the chances of contact in the turbulent flask environment are also higher, leading to greater yields. With Heterorhabditis, the above factor is not an issue, and IJ inoculum concentration, as one potential optimising factor after a protocol has been developed (Dunn et al. 2020), must be viewed in a different capacity. Therefore, a slightly different approach might need to be taken when culturing different species, with a considerably different approach being required to be taken when culturing an EPN from a different genus. However, though the above may be true, certain principles of mass production have been developed that are ubiquitous for all EPNs.
Previous work by the same authors (Dunn et al. 2020, 2022), in terms of mass culturing Steinernema jeffreyense and Steinernema yirgalemense, as guided by the work of Ferreira et al. (2014a, 2016), have paved the way for the development of simple mass production techniques in South Africa, that can be applied to all EPN species, with it being of great use to new research teams in the country, and to provide large enough volumes of EPNs for large-scale field trials. Though Ferreira et al. (2014a) successfully mass-produced H. zealandica (associated with Photorhabdus heterorhabditis, subsp. heterorhabditis), the techniques and methods developed have been modified by Dunn et al. (2020, 2022), with them not previously having been attempted with a Heterorhabditis zealandica associated with Photorhabdus thracensis (Booysen et al. 2022).
This study is the first attempt at mass-producing a South African isolate of the nematode, H. zealandica and its bacterial symbiont P. thracensis. The focus of the study was to apply the developed technique of EPN production to a Heterorhabditis species, ensuring that the in vitro flasks for this isolate of H. zealandica were free of unwanted microbes, and to also obtain high recovery and high numbers of hermaphrodites in the first few days of the process, using different proteins.
METHODS AND MATERIALS
Isolation of nematode and symbiotic bacteria
Heterorhabditis zealandica isolate MJ2C isolate was found in a citrus orchard in the Hex River Valley, Western Cape, South Africa and verified using molecular techniques (OK287289) (James et al. 2018). After identification, H. zealandica IJs were maintained and recycled, using the modified White trap method (Woodring and Kaya 1988) using Galleria mellonella L. (Lepidoptera: Pyralidae) as the bait insect. The IJs were subsequently harvested and stored in horizontally oriented well-ventilated culture flasks at 14 °C, as part of the Stellenbosch University nematode collection. The isolate of H. zealandica is referred to as the 'green' H. zealandica MJ2C, because of the colour of the G. mellonella larvae cadaver infected by the isolate, compared to the 'blue' H. zealandica SF41 (Booysen et al. 2022) that was first mass-produced by Ferreira et al. (2014a).
Photorhabdus thracensis was isolated from the haemolymph of the last instar of G. mellonella. The G. mellonella larvae were inoculated with IJs of the H. zealandica and incubated for 18 h at 25 °C. Individual G. mellonella larvae were dipped in a 75% ethanol solution, to sterilise the surface of the larvae under a laminar flow. The larvae were then held between forefinger and thumb and punctured behind one of the prolegs, with a single-use sterile needle. The droplet of haemolymph was dripped onto a nutrient agar plate, with bromothymol as a dying agent (NBTA) (8 g nutrient broth; 15 g agar; 0.25 g bromothymol blue; 1 litre distilled H2O, and 0.04 g triphenyltetrazolium chloride) and streaked using a sterile streaking rod. The agar plate was then placed in an incubator at 28 °C for 48 h, from which a single colony was removed using a sterile streaking rod under a laminar flow and added to 30 ml of tryptic soy broth (TSB) in 250-ml Erlenmeyer flasks. The flasks were then incubated in an incubating growth chamber (IncoShake Labotec) at 140 rpm at 30 °C for 48 h. After 48 h, sterile glycerol was added to the Erlenmeyer flasks as a cryopreserving agent (15% glycerol v/v; 4.5 ml) and stirred vigorously to ensure homogeneity. A 1-ml sample was then pipetted into a 1.5-ml Eppendorf tube and stored in a -80 °C freezer (Kaya and Stock 1997). The bacteria were verified and confirmed as being P. thracensis (Booysen et al. 2022). The stock culture, which was used throughout the study, and new bacteria were cultured every few months, to maintain stock volumes, or when stocks were depleted.
Stock culture development of in vitro liquid cultures of H. zealandica
Heterorhabditis zealandica were sterilised using the method developed by Dunn et al. (2020), a modified method of Lunau et al. (1993) and Ferreira et al (2014a). A 200-μl sample of P. thracensis from frozen stock cultures was inoculated onto a Wouts agar plate (16 g nutrient agar [BioLab, Merck]; 12 g agar bacteriological No. 2; 5 g olive oil, and 1000 ml distilled water) and spread as a lawn, using a sterile streaking L-shaped rod. The plates were incubated for 48 h at 30 °C in an incubating chamber. After 48 h, the plates were assessed for bacterial growth under a laminar flow chamber. IJs from stock culture flasks were washed with distilled water by means of pipetting 1 ml of the nematode solution into a 1.5-ml Eppendorf tube and centrifuging for 2 min at 10 000 rpm. This process was repeated twice.
The IJs were then inoculated onto the bacterial lawn Wouts plates and placed in an incubating chamber at 25 °C for 3-4 days, or until hermaphrodites were present in large quantities and no endotokia matricide or juveniles could be seen. The females were then washed from the plates, using distilled water and a 212-μl sieve, to remove unwanted debris from the agar plates. The hermaphrodites were then placed in a glass beaker and set aside to settle at the bottom of the flask. The hermaphrodites were inoculated into Eppendorf tubes at high concentrations of nematodes. The tubes were then centrifuged at 10 000 rpm and the supernatant removed. The hermaphrodites were macerated and crushed, using a macerating rod, in a pestle-and-mortar fashion, until the hermaphrodite bodies had been completely broken down and the eggs had been exposed. The tubes were centrifuged again at 10 000 rpm for 2 min and the supernatant removed. The procedure was repeated twice. After the supernatant was removed, a 1-ml sample of sterile annexing solution was added to each tube made up of sodium hydroxide (NaOH, AnalaR, BDH Chemicals Ltd) and sodium hypochlorite (Jik or household bleach). The tubes were then slowly turned up and over in a steady oscillating moment for 5-6 min. The tubes were placed back in the centrifuge at 10 000 rpm for another 2 min, with the supernatant then being removed, and 1 ml of sterilised distilled water being added to each tube. The process was repeated twice, until the annexing solution had been sufficiently diluted or removed, with a small droplet remaining behind with the eggs. The sterilised eggs were then added to 24-well plates previously inoculated with TSB, wrapped with parafilm, and incubated at 25 °C for 3 days.
After 3 days, the well plates were assessed for contamination, and for whether the eggs had hatched. A well was considered uncontaminated if the TSB was still clear, and if the juvenile nematodes were alive and moving. The content of the wells was then inoculated into 30 ml nutrient-complex media (15.0 g yeast extract [Sigma Life Science]; 20.0 g protein source [egg yolk powder]; 4.0 g NaCl [AnalaR, BDH Ltd]; 0.35 g KCl [AnalaR, Hopkin and William Ltd, England]; 0.15 g CaCl2 [Merck]; 0.1 g MgSo4 [PAL Chemicals] and 36 ml canola oil [Canola Oil, SPAR South Africa (Pty) Ltd)] per litre of water) in 250-ml Erlenmeyer flasks, and inoculated with 600 μl (2% v/v) of frozen and thawed P. thracensis, 48 h prior to the nematode inoculation. The flasks were placed in an incubating chamber (IncoShake) at 25 °C at 140 rpm. The flasks were observed daily, by means of tilting the flask, to enable visual assessment of nematode growth and to confirm that the inoculated J1 and J2 nematodes were developing. Samples were also taken every few days, to enable assessment of the different life stages. Due to a small initial population, the nematodes went through multiple generations until the food source was depleted, after which newly formed IJs began to appear after approximately a month. However, the population was highly unsynchronised, with many different life stages being present, including unfertilised second-generation females, as well as second-generation males. Thus, new in vitro liquid flasks were made up and inoculated with the mixed population, which was repeated thrice, until a flask with only IJs was formed, the contents of which were used as inoculum for the follow-up experiments.
In vitro liquid culture optimisation of H. zealandica
After a stock culture of IJs was established, optimisation and life cycle assessment trials could begin. In short, the nematodes were cultured using one of two treatments: soy powder protein or dried egg yolk powder protein. The TSB was inoculated with 200 μl of the symbiotic bacteria, P. thracensis, and cultured at 30 °C for 48 h at 140 rpm in an incubating chamber (IncoShake, Labotec). After 48 h, flasks of nematode nutrient complex media, as described above, were inoculated with 600 μl (2% v/v) of P. thracensis. The flask's nutrient medium differed, in the form either of soy powder protein or the dried egg yolk powder, at the same quantities of 20.0 g per litre, being used as experimental treatments of protein source. The treatment flasks were cultured at 30 °C for 48 h at 140 rpm on an IncoShake (Labotec). After 48 h, approximately 5000 IJs/ml (150 000 IJs) from stored stock cultures were inoculated into each flask. Each treatment had nine flasks (n = 18), and the experiment was repeated on a different test date.
Number of IJs recovered and J3s/J4s and hermaphrodites produced
The flasks were assessed for recovery, based on the presence of recovered or activated IJs that had developed into different life stages (J3/J4 and hermaphrodites) for the first 3 days after
IJ inoculation, with this being used as a metric for the optimal protein source. On all 3 days after IJ inoculation, a 200μl sample was taken from each flask and diluted accordingly in distilled water, until a suitable or countable number of nematodes was present. A 1-ml sample was twice taken from the diluted solution, with counting being done under a dissecting microscope (Leica), using a counting chamber. Recovery was measured as a percentage on days 1, 2 and 3, as the number of recovered individuals divided by the total number of nematodes present in solution (Strauch and Ehlers 1998). Recovered J3s/ J4s and hermaphrodites are easily distinguishable from IJs, by the loss of the outer cuticle and the presence of the vulva of the hermaphrodite.
Final yield for protein treatments
After 30 days of incubation, a 200μl sample was taken from each flask and diluted in distilled water, to count the number of IJ was present. A 1-ml sample was twice taken from the diluted solution, with counting being done under a dissecting microscope, using a counting chamber.
Statistical analysis
The IJ recovery rate presented was a mean percentage, with the number of J3/J4 and hermaphrodite-stage nematodes presented as the total number of each life stage in 1 ml of complex media solution. All data was analysed and processed using the statistical software program, STATISTICA, version 14 (StatSoft Inc. 2016), using a mixed-model ANOVA and least-square means, and a Fishers LSD test for significance.
RESULTS
Number of IJs recovered
When analysing the impact of protein source treatments on the IJ recovery of H. zealandica over 3 days after IJ inoculation, pooling the two trials, and treating them as blocks, no significant difference was detected between the two protein source treatments (ANOVA: F2, 102 = 2.4823; ρ = 0.088). However, when analysing the recovery data on each day after IJ inoculation for each treatment, a significant difference could be seen. On day 1, no significant difference was detected between the two treatments in terms of recovery (ρ = 0.26). However, by day 2 and day 3, a significant difference was detected between the two treatments (ρ < 0.001) (Figure 1). By day 3, the recovery of the egg protein had not increased substantially from day 1 and did not show the large increase in recovery as seen with the soy powder treatment. However, by day 3, the recovery of the soy powder treatment had begun to decrease. Moreover, the maximum recovery rate of the soy powder treatment was 54%, whereas the egg protein only reached a recovery maximum of 25%.
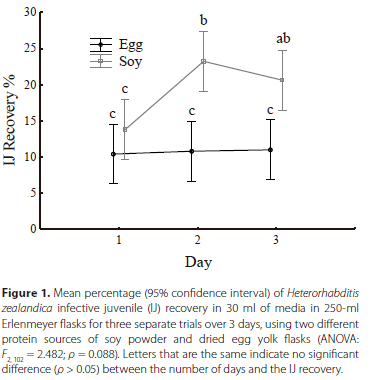
Number of J3s/J4s produced
When analysing the main effect of protein source on the production of recovered J3/J4 nematodes over 3 days, no significant difference was detected between the two treatments (F = 3.7170; ρ = 0.28). However, when analysing the number of J4/J4 nematodes produced daily, significant differences were detected. On day 1, a significant difference was detected between the two treatments, in terms of the total number of J3/J4 nematodes present in 1 ml of media (ρ = 0.032), with the soy containing more individuals. By day 2, the number of J3/J4 nematodes showed a significant difference (ρ < 0.001) between treatments. In contrast, by day 3, the number of J3/J4 nematodes had decreased, as many would by this stage, have formed hermaphrodites, as shown by the large increase in the number of hermaphrodites on day 3 (Figure 2). On day 3 for both treatments, no significant difference was detected (ρ = 0.21) between the treatments. On day 1, when the highest number of J3/J4 nematodes was present for both treatments, the soy powder treatment obtained a maximum of 1450 J3/J4 nematodes/ml, whereas the egg yolk produced only a maximum of 850 J3/J4 nematodes/ml (Table 1).
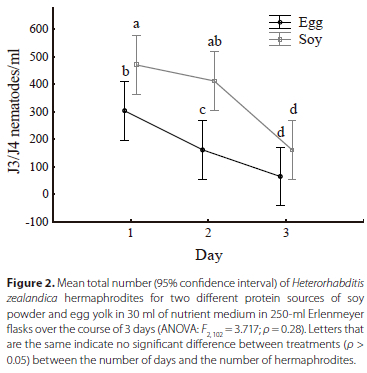
Number of hermaphrodites produced
When analysing the main effects of protein source on the production of hermaphrodites over 3 days after IJ inoculation, no overall significant difference was seen between the two treatments. However, when analysed daily, a significant difference between the two protein source treatments in terms of the number of hermaphrodites produced (Figure 3). On day 1, no significant difference was detected between the treatments, as no hermaphrodites were present in solution for all the flasks across all trials. By day 2, the recovered IJs that had formed J3/J4 stage nematodes on day 1 now began to develop into hermaphrodites, with a significant difference being detected between the two treatments, with soy giving the highest numbers of hermaphrodites. Moreover, by day 3, a significant difference was also detected between the two protein source treatments, with the soy powder producing significantly more hermaphrodites (ρ < 0.001). Additionally, the soy powder produced a maximum of 1 550 hermaphrodites/ml, compared to the maximum of 600 hermaphrodites/ml produced for the egg yolk protein (Table 1). An important factor observed in the H. zealandica flask production during the recovery process was the large number of IJs that start crawling out of both types of medium along the glass flask, which might have impacted on the recovery data. These IJs eventually moved back into the media further disrupting the process (Figure 4). Moreover, Figure 4 shows the IJs starting to crawl from the flask nearing the end of production from day 20 to 30.
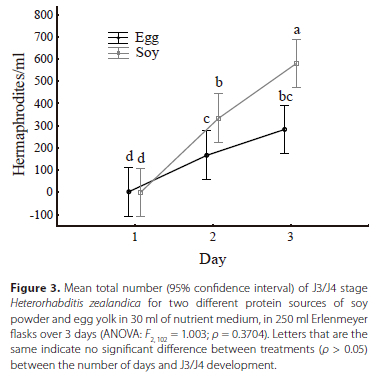
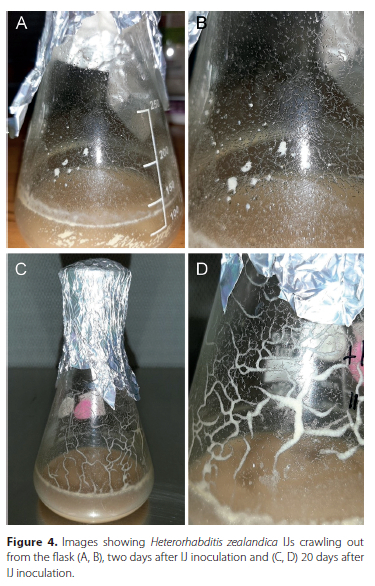
Final yield for protein treatment
The yields of IJs for both soy and egg yolk reached 120 000 to 150 000 IJs/ml after 30 days. However, the final population of nematodes unfortunately contained small numbers of other life stages such as unwanted second-generation females and males.
DISCUSSION
The in vitro liquid mass production methods for EPNs can differ between the genera Heterorhabditis and Steinernema, with them even possibly differing between species in the same genera (Ehlers 2001; Hirao and Ehlers 2009, 2010). Each new species requires thorough optimisation experiments to increase the recovery of the inoculated IJs, and to obtain high yields within a short period of time. Furthermore, the EPN in vitro liquid culture method has multiple technical pre-flask methods that contribute to the production of the final mass-produced nematodes, like the nematode sterilisation and bacteria extraction techniques (Lunau et al. 1993; Ehlers 2001; Ferreira et al. 2014b), and these methods can differ greatly between different research laboratories. Additionally, the source of ingredients for the dietary medium for the liquid culture process is likely to differ between research laboratories, due to the nature of different ingredient suppliers in different countries. Thus, it is important for each laboratory to establish standard protocols of production that work for their specific nematode isolate. After these basic protocols have been developed, that allow the different research teams to rapidly isolate and culture new species, only then should the optimisation phase begin. The current study demonstrated that a universal protocol for the use of in vitro liquid flask culture of EPNs could be established that can be used for multiple species.
Dunn et al. (2020, 2022) developed a basic protocol of mass production S. jeffreyense and S. yirgalemense, which was based on the work of Ferreira et al. (2014a, 2016). Ferreira et al. (2014a) mass-produced a Heterorhabditis zealandica SF41 (also known as the 'blue' isolate) that was associated with Photorhabdus heterorhabditis subsp. heterorhabditis, while the present study, which used the 'green' H. zealandica MJ2C, which is associated with P. thracensis (Booysen et al. 2022). The difference in symbiotic bacteria can have a major impact on the nematode, such as decreased insect pathogenicity. However, from a mass production point of view, such a phenomenon has consequences for such aspects as correct culture temperature and secondary metabolite production, leading to recovery (Strauch and Ehlers 1998; Ehlers 2001). Thus, the H. zealandica isolate would need to be treated as an entirely unique biological combination being mass-produced, as the bacteria is the key to successful EPN in vitro liquid mass production.
The results described above indicate that the mass production techniques developed for S. jeffreyense and S. yirgalemense apply to Heterorhabditis species. The bacteria extraction and egg sterilisation techniques remained the same, resulting in stock flask cultures that could be stored for experimental use (Ehlers 2001). However, the subtle difference encountered in the culturing of the Heterorhabditis species was the difficulty in establishing these stock cultures because of their tendency to create the amphimictic reproductive phase that usually does not result in the production of offspring (Strauch 1994; Ehlers 2001). After the initial egg sterilisation process, it took approximately 6 months to establish a nematode flask population of over 95% IJs to be used as an inoculum for all other flasks, because H. zealandica went through multiple generations within the flask after the inoculation of the sterilised J1 and J2 nematodes from the egg extraction process, until all their food was depleted. Compared with the process of stock culture establishment with the Steinernema species, the process was far more difficult. The difficulty lies in the copulation methods of the nematodes involved (Strauch et al. 1994).
After the first generation, during which hermaphrodites are present, the second generation of adults produce both males and females, which require copulation to produce new progeny (Poinar 1975). Though the Steinernema species also possess males and females, it is easier for the male nematode to fertilise the female, because it wraps itself around the female tightly, using a helix-type copulation (Strauch et al. 1994), especially within the shaking flask environment. Thus, the mating technique used allows the Steinernema male to wrap itself around the female and successfully fertilise the female. In contrast, the Heterorhabditis male lies along the female body longitudinally, which is also referred to as the 'y' or 'λ', or lambda style of mating (Strauch et al. 1994). As this technique results in the male being only loosely attached to the female, the shaking flask environment can pull the two nematodes apart, thus preventing fertilisation. The process results in many unfertilised females being present in the solution, which disrupts the in vitro liquid culture process, by means of creating mixed populations, as the females and males stay in solution for extended periods, and a clean IJ stock culture is not produced.
To overcome the problem, it is essential to inoculate many sterilised J1 and J2 nematodes at the start of the stock culture development process (Lunau et al. 1993; Strauch et al. 1994; Ehlers 2001), as doing so will prevent the creation of multiple generations. Once a population of IJs is established within the mixed population of nematodes, sub cultured flasks can be made (Ehlers 2001). It is recommended that new flasks be inoculated with a high concentration of IJs (not nematodes in total) from the mixed population, which will mean inoculating some unfertilised females (Strauch et al. 1994; Ehlers 2001), as well as males, which are unable to copulate, and they will have a negligible impact on the process overall. Over time, and with multiple subcultures, a stock culture flask of pure IJs, without other life-stages, can be developed, as was shown in the current study in relation to H. zealandica. A stock culture flask containing approximately 100 000 IJs/ml was developed and used for all subsequent experiments. Usually, only once a suitable IJ stock culture has been developed can the in vitro liquid mass production optimisation process begin.
As with Steinernema species, the most important aspect of EPN mass production is the recovery of the IJs. If the recovery is too low, a second generation is almost guaranteed to occur and this results in an extended process and an unsynchronised population (Strauch et al. 1994). Thus it is important, firstly, to focus on the recovery of the inoculated IJs, before redirecting one's attention to other aspects of the process. With increased recovery, more first-generation J3s/J4s and hermaphrodites that feed on the available food source will be produced, resulting in more juveniles being produced, and, thus, the likelihood of further generations will decrease, as the reduced food availability is likely to starve the feeding nematodes and encourage IJ formation, thus discouraging the development of a new generation (Hirao and Ehlers 2010).
In the present study, the impact of two protein sources on H. zealandica IJ recovery was assessed, as a means of increasing recovery and producing more adult nematodes. Studies investigating the in vitro liquid culture of other Heterorhabditis species (either directly investigating the soy protein source, or simply using it as the base protein source in the study) has shown that the use of soy-based protein tends to result in high recovery rates and yields of Heterorhabditis species (Gil et al. 2002; Cho et al. 2011), which indicates that Photorhabdus/Heterorhabditis symbiosis might have an affinity for soy-based proteins.
Noteworthily, in the Gil et al. (2002) study, the EPN being studied, namely H. bacteriophora, only achieved high yields in the presence of canola oil, although its recovery was unaffected.
Dunn et al. (2022) (when directly investigating the impact of the protein source) showed that the protein source can significantly impact the mass production of S. yirgalemense, whereby an egg yolk protein achieved far greater yields than that of soy powder. However, this was not seen with S. jeffreyense, which preferred soy protein. The above finding indicates that the optimal protein source must be established for each new species being mass-produced and was thus the first question asked regarding H. zealandica mass production; does egg yolk or soy protein provide better recovery of this species?
The results demonstrated that a simple change to the nematode complex nutrient medium can have a large impact on the growth and development of the EPNs. As was the case with other Heterorhabditis species, protein source has an impact on the nematode growth and development of H. zealandica. In contrast to egg yolk being the superior protein source for S. yirgalemense, H. zealandica seems to prefer soy powder. The reasons for this difference are unclear but a prevailing theory is that the symbiotic bacteria can digest the soy protein more easily than it does the egg yolk, although the opposite might be true in the case of other species. The above reiterates the point that each new species being mass-produced must be subjected to optimisation experiments, even if they have shown certain results in other species. In this study, a clear difference in recovery was seen between soy and egg yolk, with the use of soy powder leading to a 10% increase in the number of IJs to recover, which, in turn, produced more hermaphrodites than were obtained with the use of egg yolk.
When both protein treatments were used, the recovery was low, which resulted in a second generation developing in all the flasks used, which extended the process time up to 25-30 days, with an unsynchronised and mixed population. Due to the above, the yields were not used as a metric for optimisation, although the yields of IJs did reach 1.2 χ 105-1.5 χ 105 IJs/ml after 30 days within a mixed population. The yields obtained, although being comparable to those of other EPN species, were low compared to some reports, like that of Ehlers et al. (2000), in terms of which a mean yield of 4.57 χ 105 IJs/ml and a maximum of 6.48 χ 105 IJs/ml was achieved for Heterorhabditis indica. Interestingly, Ehlers et al. (2000) only achieved a recovery rate of a maximum of 20%, which resembled the extent of recovery achieved in the current study. Other studies have achieved much higher recovery, but lower yields, than did the H. indica study of Ehlers et al. (2000). Examples of such studies are those of Gil et al. (2002), whereby high recovery was achieved (up to 60% in some cases), with yields of up to 3.6 χ 105 IJs/ml of H. bacteriophora, and of Cho et al. (2011), where recovery reached approximately 75% with yields of 1.85 χ 105 IJs/ml being achieved, also for H. bacteriophora. The above indicates that recovery does not have to be that high to achieve high yields, although the relationship between recovery and yield also seems to be highly species-specific.
Further studies into the impact of recovery on H. zealandica yields will be investigated. Close attention must be paid to the contents of the nutrient medium used. As was shown in Gil et al. (2002), even when the recovery is high, it does not always result in high yields. The omission of certain nutrient media ingredients, like canola oil, can result in high recovery, but low yields, because of the role of certain ingredients or biochemicals, like lipids, play in the production of hermaphrodite offspring, and in the growth and development of the new generation of nematodes. Thus, the current results, in terms of recovery, achieved in the present study provide a suitable start for the in vitro liquid mass culture of H. zealandica, although research focusing on the increased production of offspring per hermaphrodite is warranted. Nevertheless, the results achieved in the present study indicate the potential of H. zealandica to reach even higher yields, if the recovery or hermaphrodite stage offspring production can be further increased by means of optimisation studies, which would also serve to help reduce the current extended process time.
ACKNOWLEDGEMENTS
The funding provided by Table Grape Industry (SATI), Hort Pome and Hort Stone and the South African, is gratefully acknowledged and D.G. Nel from the Centre of Statistical Consultation, Stellenbosch, South Africa, for his assistance with the statistical analysis.
AUTHOR CONTRIBUTIONS
APM and MDD conceptualised the idea for the research. MDD and APM designed the research and MDD conducted the experiments and analysed the data. MDD wrote the original draft of the article. All authors edited and approved the manuscript.
ORCID IDs
MD Dunn: https://orcid.org//0000-0002-0961-8101
AP Malan: https://orcid.org//0000-0002-9257-0312
REFERENCES
Boemare NE, Akhurst RJ, Mourant RG. 1993. DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. International Journal of Systematic and Evolutionary Bacteriology 43(2):249-255. https://doi.org/10.1099/00207713-43-2-249 [ Links ]
Booysen E, Malan AP, Dicks LMT. 2022. Colour of Heterorhabditis zealandica-infected-Galleria mellonella dependent on the Photorhabdus symbiont, with two new nematode-symbiotic associations reported. Journal of Invertebrate Pathology 189:107729. https://doi.org/10.1016/j.jip.2022.107729 [ Links ]
Cho CH, Whang KS, Gaugler R, Yoo SK. 2011. Submerged monoxenic culture medium development for Heterorhabditis bacteriophora and its symbiotic bacterium Photorhabdus luminescens: protein sources. Journal of Microbiology and Biotechnology 21(8):869-873. https://doi.org/10.4014/jmb.1010.10055 [ Links ]
Dunn MD, Belur PD, Malan AP. 2020. In vitro liquid culture and optimization of Steinernema jeffreyense, using shake flasks. Biocontrol 65:223-233. https://doi.org/10.1007/s10526-019-09977-7 [ Links ]
Dunn MD, Belur PD, Malan AP. 2021. A review of the in vitro liquid mass culture of entomopathogenic nematodes. Biocontrol Science and Technology 31:1-21. https://doi.org/10.1080/09583157.2020.1837072 [ Links ]
Dunn MD, Belur PD, Malan SP. 2022. Development of cost-effective media for the in vitro liquid culture of entomopathogenic nematodes. Nematology24(7):763-775. https://doi.org/10.1163/15685411-bja10166 [ Links ]
Ehlers R-U, Niemann I, Hollmer S, Strauch O, Jende D, Shanmugasundaram M, Mehta UK, Easwaramoorthy SK, Burnell A. 2000. Mass production potential of the bacto-helminthic biocontrol complex Heterorhabditis indica-Photorhabdus luminescens. Biocontrol Science and Technology 10(5):607-616. https://doi.org/10.1080/095831500750016406 [ Links ]
Ehlers RU. 2001. Mass production of entomopathogenic nematodes for plant protection. Applied Microbiology Biotechnology 56:623-633. https://doi.org/10.1007/s002530100711 [ Links ]
Ferreira T, Addison MF, Malan AP. 2014. In vitro liquid culture of a South African isolate of Heterorhabditis zealandica for the control of insect pests. African Entomology 22(1):80-92. https://doi.org/10.4001/003.022.0114 [ Links ]
Ferreira T, Addison MF, Malan AP. 2016. Development and population dynamics ofSteinernemayirgalemense(Rhabditida: Steinernematidae) and growth characteristics of its associated Xenorhabdus indica symbiont in liquid culture. Journal of Helminthology 90(3):364-371. https://doi.org/10.1017/S0022149X15000450 [ Links ]
Forst S, Nealson K. 1996. Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp and Photorhabdus spp. Microbiology Reviews 60(1): 21-43 [ Links ]
Gil G, Choo H, Gaugler R. 2002. Enhancement of entomopathogenic nematode production in in-vitro liquid culture of Heterorhabditis bacteriophora by fed-batch culture with glucose supplementation. Applied Microbiology Biotechnology 58(6):751-755. https://doi.org/10.1007/s00253-002-0956-1 [ Links ]
Grewal PS, Lewis EE, Gaugler R, Campbell JF. 1994. Host finding behaviour as a predictor of foraging strategy in entomopathogenic nematodes. Parasitology 108(2):207-215. https://doi.org/10.1017s003118200006830x [ Links ]
Hirao A, Ehlers RU. 2009. Influence of cell density and phase variants of bacterial symbionts (Xenorhabdus spp.) on dauer juvenile recovery and development of biocontrol nematodes Steinernema carpocapsae and S. feltiae (Nematoda: Rhabditida). Applied Microbiology Biotechnology 84:77-85. https://doi.org/10.1007/s00253-009-1961-4 [ Links ]
Hirao A, Ehlers RU. 2010. Influence of inoculum density on population dynamics and dauer juvenile yields in liquid culture of biocontrol nematodes Steinernema carpocapsae and S. feltiae (Nematoda: Rhabditida). Applied Microbiology Biotechnology 85: 507-515. [ Links ]
Hominick WM. 2002. Biogeography. In: Gaugler R (ed), Entomopathogenic Nematology. Wallingford: CABI. pp 115-143 . [ Links ]
James M, Malan AP, Addison P. 2018. Surveying and screening South African entomopathogenic nematodes for the control of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann). Crop Protection 105:41-48. [ Links ]
Kaya HK, Stock S. 1997. Techniques in insect nematology. In: Lacey L (ed), Manual of Techniques in Insect Pathology. San Diego: Academic Press. pp. 281-324. https://doi.org/10.1016/B978-012432555-5/50016 [ Links ]
Kaya HK, Aguillera MM, Alumai A, Choo HY, Torre M, Fodor A, et al. 2006. Status of entomopathogenic nematodes and their symbiotic bacteria from selected countries or regions of the world. Biological Control 38:134-155. [ Links ]
Lewis EE, Gaugler R, Harrison R. 1992. Entomopathogenic nematode host finding: response to host cues by cruise and ambush foragers. Parasitology 105:309-315. [ Links ]
Lunau S, Stoessel S, Schmidt-Peisker AJ, Ehlers R-U. 1993. Establishment of monoxenic inocula for scaling up in vitro cultures of the entomopathogenic nematodes Steinernema spp. and Heterorhabditis. Nematology 39:385-399. [ Links ]
Peters A, Ehlers R-D. 1994. Susceptibility of leatherjackets (Tipula paludosa and T. oleracea; Tipulidae: Nematocera) to the entomopathogenic nematode Steinernema feltiae. Journal of Invertebrate Pathology 63:163-171. [ Links ]
Poinar GO. 1975. Description and biology of a new parasitic rhabditoid Heterorhabditis bacteriophora n. gen., n. sp. Nematology 21:463-470. [ Links ]
Poinar GO. 1990. Biology and taxonomy of Steinernematidae and Heterorhabditidae. In: Gaugler R, Kaya HK (eds), Entomopathogenic Nematodes in Biological Control. Boca Raton: CRC Press. pp 23-61. [ Links ]
StatSoft Inc. 2016. Dell Statistica (data analysis software system), version 14. http://www.statsoft.com.
Strauch O, Stoessel S, Ehlers R-U. 1994. Culture conditions define automictic or amphimictic in entomopathogenic rhabditid nematodes of the genus Heterorhabditis. Fundamental and Applied Nematology 17(6):572-582. [ Links ]
Strauch O, Ehlers RU. 1998. Food signal production of Photorhabdus luminescens inducing the recovery of entomopathogenic nematodes Heterorhabditis spp. in liquid culture. Applied Microbiology Biotechnology 50:369-374. https://doi.org/10.1007/s002530051306 [ Links ]
Torr P, Spiridonov SE, Heritage S, Wilson MJ. 2007. Habitat associations of two entomopathogenic nematodes: a quantitative study using realtime quantitative polymerase chain reactions. Journal Animal Ecology 76:238-245. https://doi.org/10.1111/j.1365-2656.2006.01196.x [ Links ]
Warrior P. 2000. Living systems as natural crop-protection agents. Pest Management Science 56:681-687. https://doi.org/10.1002/1526-4998(200008)56:8<681::AID-PS199>3.0.CO;2-S [ Links ]
Woodring JL, Kaya HK. 1988. Steinernematid and Heterorhabditid Nematodes: A Handbook of Techniques. Southern Cooperative Series Bulletin 331. Fayetteville, Arkansas Agricultural Experimental Station.
Yoo SK, Brown I, Gaugler R. 2000. Liquid media development for Heterorhabditis bacteriophora: lipid source and concentration. Applied Microbiology Biotechnology 54:759-763. https://doi.org/10.1007/s002530000478 [ Links ]
 Correspondence:
Correspondence:
Murray D. Dunn
Email: mdunn94@sun.ac.za
Received: 16 May 2023
Accepted: 16 August 2023














