Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a11581
RESEARCH ARTICLE
Identification and virulence screening of fungal and bacterial entomophathogens of the edible long-horned grasshopper Ruspolia differens (Orthoptera: Tettigoniidae) from Uganda
Alfonce LeonardI, II, III; James P. EgonyuI; Fathiya M. KhamisI; Chrysantus M.TangaI; Sunday EkesiI; Samuel KyamanywaII; Sevgan SubramanianI
IInternational Centre of Insect Physiology and Ecology, Nairobi, Kenya
IIDepartment of Agricultural Production, Makerere University, Kampala, Uganda
IIITanzania Agricultural Research Institute (TARI)-Ukiriguru, Mwanza, Tanzania
ABSTRACT
Natural enemies are major challenges in laboratory rearing of grasshoppers, but the identity and virulence of these towards the edible long-horned grasshopper Ruspolia differens (Serville) is scarcely known. In this study, fungi and bacteria were isolated from R. differens collected from Mbarara, Masaka, Hoima, Kampala and Kabale districts in Uganda in 2018, cultured on standard microbial media, identified using molecular techniques and screened for virulence against the insect in laboratory bioassays. Fourteen and nine species of fungi and bacteria were isolated from R. differens, respectively, with the number of isolates varying based on collection site. The most prevalent entomopathogenic fungal species were Aspergillus flavus Link (27.3%), Fusarium equiseti (Corda) (24.2%), Mucorfragilis Fresen (12.1%), Clonostachys rosea (Link) (6.0%) and Aspergillus tamarii Kita (6.0%); whereas the most prevalent bacterial isolates were Serratia marcescens Bizio (38.10%), Bacillus thuringiensis (Berliner) (14.3%) and Enterobacter cloacae (Jordan) (14.3%). Nine of the fungal species namely Clavispora lusitaniae Rodrigues de Miranda, Lichtheimia corymbifera (Cohn), Trichoderma koningii Oudem, F. equiseti, M. fragilis, Aspergillus niger van Tieghem, Epicoccum sorghinum (Saccardo), C. rosea, Penicillium commune Charles Thorn; and five bacterial species (Proteus penneri Hickman, S. marcescens, B. thuringiensis, Staphylococcus sciuri Kloos and Enterococcus faecalis (Andrewes and Horder)) were ~5-7-fold and ~4-5-fold, more lethal to third instars of R. differens than untreated controls, respectively. This study is the first to report C. lusitaniae, Exserohilum mcginnis Padhye and Ajello, E. sorghinum, P. penneri and E. cloacae as insect pathogens. The results suggest a need to quarantine field collected R. differens before introducing them into the insectary, as well as performing antimicrobial practices during rearing of the insect to prevent entomopathogen-based mortality.
Keywords: mass rearing, molecular analysis, mortality, natural enemies, pathogenicity
INTRODUCTION
The long-horned grasshopper, Ruspolia differens (Serville) (Orthoptera: Tettigoniidae) is an important food in East Africa (Agea et al. 2008; Kinyuru et al. 2011; Kinyuru et al. 2012). The nutritional composition of R. differens is of higher quality than that of commonly consumed insects, animals and plants (Ssepuuya et al. 2016). Ruspolia differens is a good source of protein (43-44%), fat (46-48%), ash (3%), and fibre (4-5%) (Kinyuru et al. 2011). Processed (deep-fried, toasted or smoked) ready to eat R. differens are reportedly either free from food-borne pathogens or contain them at safe levels (Ng'ang'a et al. 2018; Grabowsk and Klein2017; Labu et al. 2021). The value of R. differens in Kampala, Uganda in 2008 was US$ 2.8 per kg, which was 40% higher than the value of beef (Agea et al. 2008).
Ruspolia differens swarms during rainy seasons and exists in a non-swarming solitary phase during the dry seasons (Matojo and Yarro 2013). Seasonality of R. differens swarms limits availability and reliability of its supply (Agea et al. 2008). For this reason, there have been efforts to develop protocols for mass rearing of R. differens (Malinga et al. 2018a; Malinga et al. 2018b; Lehtovaara et al. 2015). However, low survival rate of ~38% is a major challenge in R. differens mass rearing (Malinga et al. 2018b). Detailed investigations on the factors fueling R. differens death during rearing are therefore urgently warranted. Some factors that affect R. differens survival have been investigated. For example, Leonard et al. (2021) and Lehtovaara et al. (2015) have reported that temperature greatly influences survival of R. differens, with 27-30 °C reported as optimum temperatures for mass rearing of the insects. Cannibalism is another factor that obstructs mass production of R. differens (Hartley 1967), contributing up to 49% of R. differens mortality during laboratory rearing (Egonyu et al. 2021). However, the role of natural enemies in the mortality of R. differens has been scarcely investigated.
Entomopathogenic bacteria and fungi have been reported as key challenges in laboratory rearing ofgrasshopper species (Bailey and McCrea 1978; Hinks and Erlandson 1994). The insects get infected by these entomopathogens mainly through introducing fresh field collections into established colonies and feeding them on infested materials (Hinks and Erlandson 1994). Shah et al. (1997) reported numerous entomopathogenic fungal isolates from different short-horned grasshopper species collected from the field, including seven Metarhizium anisopliae (Metchnikoff) Sorokin, 121 Metarhizium flavoviride Gams and Roszypal, 33 Beauyeria bassiana (Bals.-Criv.) Vuill and 20 Sorosporella sp. Mortalities of80-100% have been reported in the variegated grasshopper Zonocerus variegatus (L) (Orthoptera: Pyrgomorphidae) infected with B. bassiana, M. anisopliae, Aspergillus niger van Tieghem, Mucor sp. and Penicillium sp. (Balogun and Fagade 2004). Entomopathogenic bacterial species Pseudomonas aeruginosa (Schröter), Serratia marcescens Bizio and Coccobacillus acridiorum d'Herelle have been isolated from laboratory-reared desert locust Schistocerca gregaria Forskâl (Orthoptera: Acrididae) (Zelazny et al. 1997; Hinks and Erlandson 1994). Although unidentified moulds have been observed on cadavers of Homorocoryphus nitidulus (Scopoli) which was later renamed R. differens (Bailey and McCrae 1978), information on identity and virulence of entomopathogens of R. differens is scarce.
This study aimed to determine the entomopathogens of R. differens originating from the field for development of their control strategies during mass production of this edible insect. The research questions were (1) which microbial natural enemies of R. differens occur in Uganda? and (2) how virulent are these microbes against R. differens nymphs under laboratory conditions?
MATERIALS AND METHODS
Insect sampling
About 500 adult R. differens were collected in November 2018 from Mbarara, Masaka, Hoima, Kampala and Kabale districts of Uganda in the same locations described by Leonard et al. (2020). Swarming R. differens were collected between 20:00 and 21:00 h from commercial fluorescent light traps fitted to the top ofa wooden frame to attract the grasshoppers, which would then be intercepted by reflective slanting iron sheets that slide them into clean collection drums (Okia et al. 2017). At each collection site, insects were placed in aerated 60 cm χ 60 cm χ 60 cm Plexiglas cages (~100 per cage). In each cage, insects were provided with Panicum maximum (Jaq.) grass as food and transported to the International Centre of Insect Physiology and Ecology (ICIPE), Nairobi, Kenya for analysis and/ or rearing. The conditions of the rearing unit were maintained at 28 ± 1 °C, 60-70% RH and a photoperiod of 12L:12D. These insects were kept in clean aerated Plexiglas cages of the same dimensions as those used for field collection, with a stocking density of 100200 first instars per cage. The number of insects was reduced as nymphs grew to 30-50 per cage at the adult stage (Egonyu et al. 2021). Ruspolia differens in the rearing cages were fed ad libitum with maize leaves or P. maximum grass and a protein supplement made from ground dog food (Sigma Feeds Ltd, Nairobi, Kenya) and ground black soldier fly larval meal from ICIPE.
Isolation and culturing of fungi and bacteria
Grasshopper cadavers from field-collected R. differens with signs of bacterial and fungal infections such as mycosis and soft tissues were sampled from the cages. Fungal isolation involved 30, 10, 5 and 5 R. differens cadavers from Masaka, Hoima, Kabale and Mbarara, respectively; while isolation of bacteria was based on 20, 10, 4 and 3 samples from Masaka, Hoima, Mbarara and Kabale, respectively. The samples were surface sterilised with 1% sodium hypochlorite solution and approximately 30 ml of detergent (Fischer Scientific Company, New Hampshire, USA) for 5 minutes (Tuininga et al. 2009). Thereafter, the samples were submerged in 70% ethanol for 1 minute followed by rinsing with sterile distilled water (Lacey and Brooks 1997).
Cadavers for fungal isolation were longitudinally dissected into half with a sterile scalpel, and the anterior dorsal and ventral viscera were plated on Potato Dextrose Agar and Sabouraud Dextrose Agar media (Hardy Diagnostic Company, California, USA). About 0.5 g of chloramphenicol (Wellona Pharma, Nana Varachha, India) were added to 500 ml of the media before autoclaving to prevent growth of other microflora. The cultures were incubated at 25 ± 2 °C in darkness for three days. The fungal colonies from each plated cadaver were sub-cultured in the same media to obtain pure isolates of each morphotype (Opisa et al. 2018).
Isolation of bacteria was carried out by placing grasshopper samples into 1.5 ml tubes containing 1 ml of sterile water per 0.2-0.4 g of infected part of insect tissue (Lacey 2012; Chellaram and Praveen 2015). Insects were crushed within this tube using a micro pestle (Universal Medical Inc, Massachusetts, USA) and stock concentrations of each sample were plated on nutrient agar and incubated at 30 °C for 24 hours. The growth of bacterial colonies was examined and single bacterial cells were isolated using a sterile loop into nutrient agar to get pure isolates. Spores of each isolate were harvested from nutrient agar and 7.5 μl of each isolate were transferred to 50 ml lysogeny broth (LB) in sterile conical flasks and incubated in an Innova® 44 incubator shaker (Eppendorf Corporate, Hamburg, Germany) at 250 rpm and 30 °C for 48 hours.
DNA Extraction and Polymerase Chain Reaction
Fungal conidia were harvested from 2-week old sporulating cultures and placed in 1.5 ml Eppendorf tubes. DNA was extracted from fungal conidia of each isolate using ISOLATE II Plant DNA Kit from BIOLINE (Meridian Life Science Company) as per manufacturer's instructions. Bacterial cells of each isolate were harvested after 48 hours in falcon tubes and centrifuged in Eppendorf centrifuge 5810 R (Eppendorf Corporate, Hamburg, Germany) at 3900 rpm and 4 °C for 10 minutes. The pellets were gently washed twice with 45 ml distilled water and centrifuged again at 3900 rpm and 4 °C for 10 minutes. Bacterial cells were transferred into Eppendorf tubes for DNA extraction. Bacterial DNA of each isolate was extracted following the ISOLATE II Genomic DNA Kit from BIOLINE (Meridian Life Science Company) as per manufacturer's instructions.
The 16s gene for all bacterial isolates was amplified using universal primers 27F 5' AGAGTTTGATCMTGGCTCAG 3' and 1492r 5' TACCTTGTTACGACTT 3' (Suzuki and Giovannoni 1996). The ITS gene region in fungal isolates was amplified using ITS 4 5' GGAAGTAAAAGTCGTAACAAGG 3' and ITS 5 5' TCCTCCGCTTATTGATATGC 3' (Bal et al. 2016). Amplifications were conducted in 30 μ! final reaction volumes containing 5X My Taq buffer, 0.5 mol of each primer, 0.5 mM MgCl2, 0.625 U of My Taq DNA polymerase and 15 ng ml-1 of DNA template (Bioline, London, UK). Polymerase Chain Reaction (PCR) amplification was carried out in a Mastercycler nexus gradient (Eppendorf) thermal cycler programmed for initial denaturation at 95 °C for 2 minutes, followed by 40 cycles of denaturation at 95 °C for 30 seconds, 16s primers annealed at 56.4 °C and ITS primers at 59 °C for 40 seconds, and extension at 72 °C for 1 minute. The last extension step was accomplished at 72 °C for 10 minutes. PCR products were visualised on 1% agarose gels stained with ethidium bromide (EtBr) for 1 hour at 80 volts.
Sequencing, identification and phylogenetic analysis of fungal and bacterial DNA
Purification of PCR products was done using a PCR kit from BIOLINE (Meridian Life Science Company) as per the manufacturer's instructions. Purified PCR products were sent to Macrogen Europe (Amsterdam, Netherlands) for sequencing using an Applied Biosystems 3730XL sequencer. Sequences were edited using BioEdit software (Version 7.0.4) (Hall 1999) whereby forward and reverse sequences of each isolate were aligned and edited to resolve nucleotides ambiguities of the consensus sequences. Edited sequences were compared with known sequences of bacteria and fungi in the GenBank database using the nucleotide Basic Local Alignment Search Tool (BLAST) on the National Centre for Biotechnology Information (NCBI) website (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Pathogenicity of fungal isolates against third instars of Ruspolia differens
Third instars from a third R. differens generation in the laboratory colony were used in bioassay experiments. For species with more than one isolate, conidia of one isolate selected randomly were harvested from 2-week old sporulating cultures and suspended in conical flasks with 10 ml distilled water and 0.05% Triton X-100. Glass beads (φ = 3 mm) were placed in each flask and the suspension was mixed for 5 minutes using a vortex mixer at 100 rpm to homogenise the suspension and break the conidial clumps (Opisa et al. 2017). Quantification of conidial concentrations were conducted using a haemocytometer. The conidial suspensions obtained in each fungal species were adjusted to 1 χ 108 conidia/ ml for the bioassays. Each entomopathogenic fungal isolate was screened for virulence against R. differens third instars. Aliquots of conidial suspensions (10 μ!) of each fungal isolate were carefully applied under the pronotal shield of test third instars of R. differens using Exmire micro syringe (ITO Corporation, Tokyo, Japan) (Balogun and Fagade 2004). The conidial suspensions were not applied throughout the insects' body to avoid losing them during moulting. Distilled water containing only 0.05% Triton X-100 was applied to the insects used as untreated controls. Control treatments were used to correct for the effect of additional factors on the mortality of R. differens nymphs (Opisa et al. 2008). After treatment, insects were individually transferred into 6 cm depth χ 6 cm diameter containers to avoid cannibalism. Each isolate was administered to 15 test insects. Insects treated with fungal isolates were arranged in a completely randomised design and fed on materials described above. The treated insects were maintained at 28 ± 2 °C, 12L:12D and 55% RH. Nymphal mortality was recorded for 20 days (Opisa et al. 2018). Dead grasshoppers were tested for mycosis following the protocol by Opisa et al. (2018) and Balogun and Fagade (2004). Briefly, insect cadavers were surface sterilised with 70% alcohol and rinsed twice in distilled water, and kept separately in Petri dishes creased with sterile moist filter paper. Cadavers in which fungal mycosis occurred were recorded as a verification that insect mortality was caused by the test fungal isolates.
Pathogenicity of bacterial isolates against third instars of Ruspolia differens
Bacterial spores were quantified using BioSpec-min uv-visible spectrophotometer (Shimadzu Corporation, Kyoto, Japan). For species with more than one isolate, spores of one isolate selected randomly were harvested from one-day old sporulating cultures. The spores were mixed with distilled water containing 0.05% Triton X-100 in sterile conical flasks. The serial concentration obtained in most isolates was 1 χ 108 spores/ml, therefore other bacterial isolates with concentration above 1 χ 108 were diluted to have a uniform concentration in each treatment. For each bacterial species, 15 third instars of R. differens were starved for 24 hours (Opisa et al. 2018) and separately placed into 6 cm depth χ 6 cm diameter containers with feeding materials as described above. After the insects had settled on leaves, they were sprayed with 10 ml of the test spores suspension using the Burgerjon's spray tower (Burgerjon 1956) and allowed to continue feeding. Distilled water containing Triton was also sprayed to the control treatments to correct for the effect of other factors on the mortality of R. differens nymphs (Opisa et al. 2008). The experimental design was similar to the one described above on pathogenicity of entomopathogenic fungi. Nymphal mortality was recorded for 7 days (Opisa et al. 2018).
Data analysis
Data for cumulative dead third instar R. differens across treatments were analysed using R software (version 3.3.0) (R Core Team 2016) via the interface R studio (version 1.2.5).
The analyses were carried out using binomial generalised linear models (GLM) with log-link function. Over dispersion of data was assessed using the ratio of residual deviances to degrees of freedom; and corrected by fitting a quasi-binomial GLM using the MASS package (Venables and Dichmont 2004). Tukey's tests were used in pairwise multiple comparisons among treatments using emmeans package (Lenth and Lenth 2018).
RESULTS
Identification of fungal isolates
Out of 33 fungal isolates obtained from all R. differens samples, 25 were from Masaka, 7 from Hoima, 1 from Kabale and none from Mbarara and Kampala (Table 1; Supplementary Figure A1). All sequences of the fungal isolates matched with sequences deposited in the GenBank by 97 to 100%. The fungal species identified in R. differens samples from Masaka were dominated by Aspergillus flavus Link and Fusarium equiseti (Corda); whereas those from Hoima were dominated by Mucor gilis Fresen. Fusarium equiseti was the only fungal species isolated from R. differens samples from Kabale.
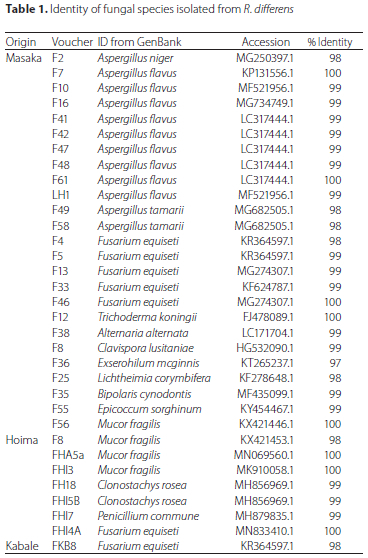
The 33 fungal isolates comprised 13 species with nine, seven, four, two and two isolates identified as A. flavus, F. equiseti, M. fragilis, Clonostachys rosea (Link), and Aspergillus tamarii Kita, respectively. Each of the other eight isolates consisted of one fungal species namely A. niger, Trichoderma koningii Oudem, Alternaria alternata (Fr.) Keissl., Clavispora lusitaniae Rodrigues de Miranda, Exserohilum mcginnis A.A. Padhye and Ajello, Lichtheimia corymbifera (Cohn) Vuill., Bipolaris cynodontis (Marignoni) Shoemaker, Epicoccum sorghinum (Saccardo) and Penicillium commune Charles Thom.
Identification of bacterial isolates
Out of 21 bacterial isolates obtained from all R. differens samples, 15 were from Masaka, five from Hoima, one from Mabara and none from Kabale and Kampala (Table 2; Supplementary Figure A2). The sequences of bacterial isolates matched with bacterial sequences deposited in the GenBank by 97 to 99%. The bacterial species identified in R. differens samples from Masaka were dominated by S. marcescens, Enterobacter cloacae (Jordan) and Proteus vulgaris Hauser; whereas Bacillus thuringiensis (Berliner) and Enterococcus faecalis (Andrewes and Horder) dominated samples from Hoima. Staphylococcus sciuri Kloos was identified from the only bacterial isolate from Mbarara.
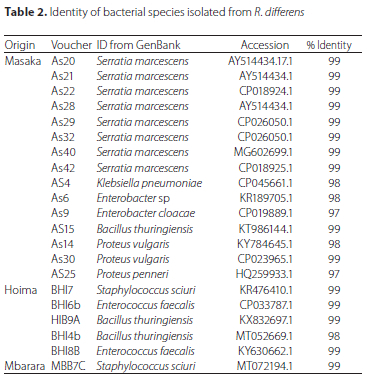
The 21 bacterial isolates comprised nine species with eight, three, two, two and two of them identified as S. marcescens, B. thuringiensis, E. faecalis, S. sciuri and Proteus vulgaris Hauser, respectively. The remaining four isolates consisted of one species each, namely E. cloacae, Enterobacter sp., Klebsiella pneumoniae (Schroeter) and Proteus penneri Hickman.
Virulence of fungal isolates to third instars of Ruspolia differens
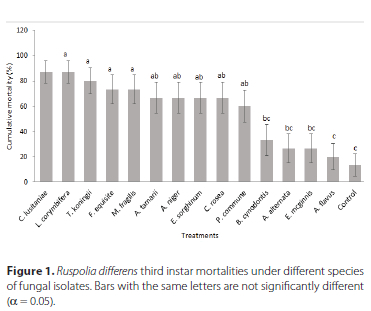
Mortality of third instars of R. differens varied significantly among fungal species tested (F = 8.60, df = 28, p < 0.001) (Figure 1). Ten fungal species, namely, L. corymbifera, L. corymbifera, C. lusitaniae, T. koningii, F. equiseti, E. sorghinum, M. fragilis, A. tamarii, A. niger, C. rosea and Penicillium commune Charles Thom caused statistically higher mortalities of R. differens nymphs than the mortality of the insects in the untreated control. The mortalities of R. differens nymphs that were caused by the other four fungal isolates were not significantly different from that of the control. Mycosis was observed in A. tamarii (3 insects), E. sorghinum (2 insects), A. niger (2 insects), T. koningii (1 insect), B. cynodontis (1 insect) and F. equiseti (1 insect) (Supplementary Figure A3).
Virulence of bacterial isolates to third instars of Ruspolia differens
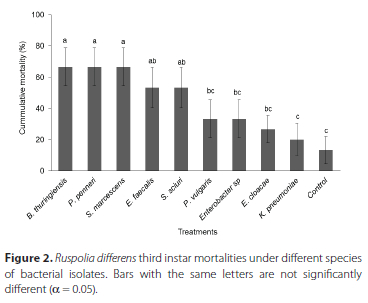
There were significant differences in virulence of bacterial species to R. differens nymphs (F = 9.08, df = 20, p = < 0.001) (Figure 2). Five bacterial species namely, P. penneri, S. marcescens, B. thuringiensis, S. sciuri and E. faecalis caused statistically higher mortalities of R. differens nymphs than that of the control. The mortalities of R. differens nymphs caused by the other four bacteria isolates were not significantly different from that of the control.
DISCUSSION
High mortality has been reported as a key challenge to artificial mass rearing of R. differens (Malinga et al. 2018b). The factors that contribute to high mortality of the insects during rearing remain obscure. Specifically, the role ofentomopathogens in causing morality of R. differens during mass production has not yet been elucidated.
We recorded the highest number of fungal (75.8%) and bacterial (71.4%) isolates from R. differens samples collected from Masaka followed by those collected in Hoima (21.2% and 23.8%), respectively. Kabale and Mbarara had the lowest number of fungal and bacterial isolates while no entomopathogenic isolates were recorded in the samples from Kampala. These results corroborate the findings by Ssepuuya et al. (2019) that raw R. differens samples marketed in Masaka had highest microbial counts compared to Kampala and Fort Portal. The results also partly concur with the findings by Labu et al. (2021) that although fungal counts in wild-harvested and traded R. differens were comparable between Masaka and Kampala during two harvesting seasons, bacterial counts in the samples were higher in Masaka than Kampala during the wetter first season. The influence of geographical location on abundance of entomopathogens could be attributed to the way grasshoppers are handled during and after collection (Ssepuuya et al. 2019).
In this study, 13 species of fungi were isolated from R. differens cadavers, some of which are known entomopathogens of grasshoppers. For example, A. flavus, A. niger, Mucor sp. and Fusarium sp. which were among the fungi isolated from R. differens were previously reported to cause 80-100% mortality of Z. variegatus (Balogun and Fagade 2004). In addition, Kumar et al. (2013) reported up to 27% mortality of seven acridid and one pyrgomorphid grasshoppers treated with A. flavus and A. niger. Other isolates have been reported as entomopathogens of insects in other orders than Orthoptera. For example, A. fluvus has been reported to kill 70-100% of all stages of the scolytid coffee twig borer, Xylosandrus compactus Eichhoff (Coleoptera: Curculionidae) (Mukasa et al. 2019). Furthermore, 45.5-82.5% mortalities were reportedly caused by C. rosea in Oncometopia tucumana (Link: Fries) and Sonesimia grossa (Link: Fries) (Hemiptera: Cicadellidae) (Toledo et al. 2006). Trichoderma koningii reportedly caused 8.3-46.6% mortality in Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) (Khaskheli et al. 2019). Alternaria alternata is known to kill 16.1-43.3% of Henosepilachna vigintioctopunctata Fabricius (Coleopteran: Coccinellidae) (Sharma et al. 2012); and 83-100% of aphids (Hemiptera: Aphididae) (Aphis fabae Scopoli, Aphis gossypii Glover, Anuraphis nerii Fonsc., Acyrthosiphon pisum Harris, Rhopalosiphum padi (L.) and Sitobion fragariae (Walk.), Uroleucon sp.) (Christias et al. 2001). Lichtheimia corymbifera was found to kill 6.5-9.5% of Bruchus bilineatopygus Pic (Coleoptera: Bruchidae) (Meshram et al. 2015). To our knowledge, R. differens entomopathogenic fungi C. lusitaniae, E. mcginnis and E. sorghinum have not yet been reported as pathogens of any insect species.
Nine bacterial species were recorded in R. differens samples during this study. Of these, S. marcescens was the most prevalent species (8 out of 21 isolates) followed by B. thuringiensis and E. cloacae. Labu et al. (2021) reported that the grasshopper appears to pick-up these microbes from commercial traps, as they were only present in R. differens samples from the traps but absent in those collected from the wild using sweep nets. These species have also been reported as entomopathogenic bacteria in various insects. For instance, S. marcescens killed up to 40% of Curculio caryae (Horn) larvae (Coleoptera: Curculionidae) (Shapiro-Ilan et al. 2004). Bacillus thuringiensis are renowned for producing toxins (Geiser et al. 1986), which have been reported to kill 69.2% of Crepidodera aurata (Marsham) (Coleoptera: Chrysomelidae) (Yaman and Ertürk 2016). Staphylococcus sciuri has been reported as an entomopathogen of C. aurata with mortality of 66.6% (Yaman and Ertürk 2016). Enterococcus faecalis has been reported to kill 100% of Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) (Youngjin et al. 2002). Proteus vulgaris is an entomopathogen of Heliothis virescens (Fabricius) (Lepidoptera: Noctuidae) with mortality of up to 100% (Miller et al. 1995). Klebsiella pneumoniae has been reported to cause up to 100% mortality of Galleria mellonella (L.) (Lepidoptera: Pyralidae) (Insua et al. 2013). To our knowledge, this is the first report of P. penneri and E. cloacae as insect pathogens.
Mycosis in R. differens cadavers treated with fungal isolates in this study was low. Similar results have been reported by Bateman et al. (1996) when the pathogenicity of numerous fungal isolates of Metarhizium and Beauveria sp. were screened against S. gregaria whereby some isolates showed non-mycosis on insect cadavers. The non-mycosis mortality could be attributed to the action of toxins produced by the isolates (Bateman et al. 1996). Low mycosis in the current study could also be attributed to factors affecting mycosis such as temperatures, relative humidity and moisture in the environment (Inglis et al. 1996; de la Rosa et al. 2000; Garrido-Jurado 2011). These factors were not controlled in this study, therefore, further studies to confirm their possible effects on mycosis of R. differens infected by the isolates would be helpful.
Comparable mortalities of R. differens nymphs treated with fungal isolates namely, L. corymbifera, C. lusitaniae, T. koningii, F. equiseti, E. sorghinum, M. fragilis, A. tamarii, A. niger, C. rosea and P. commune and those treated with bacterial isolates namely, P. penneri, S. marcescens and B. thuringiensis were significantly higher than that of the control. These entomopathogens could be the source of high R. differens mortality previously observed during R. differens rearing (Malinga et al. 2018b). For this reason, common antimicrobial practices in insect rearing such as disinfecting rearing cages with 0.5% chlorocresol or 70% alcohol, and quarantining new grasshoppers collected from the field before introducing them into an established colony (Hinks and Erlandson 1994) are recommended to reduce R. differens mortality. Mortality of R. differens nymphs differed significantly among fungal and bacterial species tested. This could be attributed to the differences in the abilities of entomopathogenic fungi to produce enzymes such as proteases, chitinases and lipases which are responsible for degrading constituents of cuticles such as protein, chitin and lipids, respectively (Khan et al. 2017) to allow easy penetration of the hyphae (Wang et al. 2005; Cho et al. 2006). The virulences among entomopathogenic bacterial species depend on their ability to produce digestive toxins and cytotoxins (Castagnola and Stock 2014). Fungal and bacterial species react differently to environmental factors such as temperature, relative humidity and UV light (Khan et al. 2017; Gonzalez et al. 2017), which might have also contributed to differences in mortality of R. differens nymphs treated with the species of fungi and bacteria tested.
CONCLUSION
The most prevalent entomopathogenic fungi in R. differens collected from different locations in Uganda were A. flavus, F. equiseti, M. fragilis, C. rosea and A. tamarii. Other than A. flavus, these isolates and five others caused mortalities of R. differens nymphs that were significantly higher than that from the untreated control. On the other hand, bacterial isolates P. penneri, S. marcescens, B. thuringiensis, S. sciuri and E. faecalis also killed significantly higher numbers of R. differens nymphs than in the control. This study reports for the first time new entomopathogenic fungi namely C. lusitaniae, E. mcginnis and E. sorghinum and bacteria namely P. penneri and E. cloacae. Quarantining newly field-collected R. differens to determine absence of entomopathogens before introducing them into the insectary, and performing antimicrobial practices during insect rearing are recommended to reduce mortality during mass rearing of the insects.
ACKNOWLEDGEMENTS
We acknowledge Fidelis Levi Ombura, Maureen Adhiambo and Miti Mathew for their technical support. Leonard, Alfonce was supported by a German Academic Exchange Service (DAAD) In-Region Postgraduate Scholarship. This work received financial support from the German Federal Ministry for Economic Cooperation and Development (BMZ) commissioned and administered through the Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) Fund for International Agricultural Research (FIA), grant number: 012345678; the BioInnovate Africa Programme (INSBIZ - Contribution ID No. 51050076); the Canadian International Development Research Centre and the Australian Centre for International Agricultural Research (Grant Number: 108866-001), the Norwegian Agency for Development Cooperation (Grant Number: RAF-3058 KEN-18/0005); the Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); the Federal Democratic Republic of Ethiopia; and the Government of the Republic of Kenya. The views expressed herein do not necessarily reflect the official opinion of the donors.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ORCID ID
James P. Egonyu - https://orcid.org/0000-0001-6297-4765
REFERENCES
Agea JG, Biryomumaisho D, Buyinza M, Nabanoga GN. 2008. Commercialization of Ruspolia nitidula (nsenene grasshoppers) in central Uganda. African Journal of Food, Agriculture, Nutrition and Development 8(3): 319-332. https://doi.org/10.4314/ajfand.v8i3.19195 [ Links ]
Bailey WJ, McCrae AWR. 1978. The general biology and phenology of swarming in the East African tettigoniid Ruspolia differens (Serville) (Orthoptera). Journal of Natural History 12(3): 259-288. https://doi.org/10.1080/00222937800770151 [ Links ]
Bal J, Yun SH, Yeo SH, Kim JM, Kim DH. 2016. Metagenomic analysis of fungal diversity in Korean traditional wheat-based fermentation starter nuruk. Food Microbiology 60: 73-83. https://doi.org/10.1016/j.fm.2016.07.002 [ Links ]
Balogun SA, Fagade OE. 2004. Entomopathogenic fungi in population of Zonocerus variegatus (L.) in Ibadan, south west, Nigeria. African Journal of Biotechnology 3(8): 382-386. https://doi.org/10.5897/AJB2004.000-2074 [ Links ]
Bateman R, Carey M, Batt D, Prior C, Abraham Y, Moore D, Jenkins N, Fenlon J.1996. Screening for virulent isolates of entomopathogenic fungi against the desert locust, Schistocerca gregaria Forskâl. Biocontrol Science and Technology 6(4): 549-560. https://doi.org/10.1080/09583159631181 [ Links ]
Burgerjon A. 1956. Pulvérisation et poudrage au laboratoire par des préparations pathogènes insecticides. Ann Epiphyt 7: 675-683. [ Links ]
Castagnola A, Stock SP. 2014. Common virulence factors and tissue targets of entomopathogenic bacteria for biological control of lepidopteran pests. Insects 5(1): 139-166. [ Links ]
Christias CH, Hatzipapas P, Dara A, Kaliafas A, Chrysanthis G. 2001. Alternaria alternata, a new pathotype pathogenic to aphids. BioControl 46(1): 105-124. https://doi.org/10.1023/A:1009930112152 [ Links ]
Cho EM, Boucias D, Keyhani NO. 2006. EST analysis of cDNA libraries from the entomopathogenic fungus Beauveria (Cordyceps) bassiana. II. Fungal cells sporulating on chitin and producing oosporein. Microbiology 152(9): 2855-2864. [ Links ]
De La Rosa W, Alatorre R, Barrera JF, Toriello C. 2000. Effect of Beauveria bassiana and Metarhizium anisopliae (Deuteromycetes) upon the coffee berry borer (Coleoptera: Scolytidae) under field conditions. Journal of Economic Entomology 93(5): 1409-1414. https://doi.org/10.1603/0022-0493-93.5.1409 [ Links ]
Egonyu JP, Miti MM, Tanga CM, Leonard A. 2021. Cannibalism, oviposition and egg development in the edible long-horned grasshopper, Ruspolia differens (Orthoptera: Tettigoniidae) under laboratory conditions. Journal of Insects as Food and Feed 7(1): 89-97. https://doi.org/10.3920/JIFF2020.0018 [ Links ]
Garrido-Jurado I, Valverde-García P, Quesada-Moraga E. 2011. Use of a multiple logistic regression model to determine the effects of soil moisture and temperature on the virulence of entomopathogenic fungi against pre-imaginal Mediterranean fruit fly Ceratitis capitata. Biological Control 59(3): 366-372. https://doi.org/10.1016/j.biocontrol.2011.09.011 [ Links ]
Geiser M, Schweitzer S, Grimm C. 1986. The hypervariable region in the genes coding for entomopathogenic crystal proteins of Bacillus thuringiensis: nucleotide sequence of the kurhd1 gene of subsp. kurstaki HD1. Gene 48(1): 109-118. https://doi.org/10.1016/0378-1119(86)90357-4 [ Links ]
Gonzalez F, Tkaczuk C, Dinu MM, Fiedler Z, Vidal S, Zchori-Fein E, Messelink GJ. 2016. New opportunities for the integration of microorganisms into biological pest control systems in greenhouse crops. Journal of Pest Science 89(2): 295-311. [ Links ]
Grabowski NT, Klein G. 2017. Microbiology of processed edible insect products-Results of a preliminary survey. International Journal of Food Microbiology 243: 103-107. https://doi.org/10.1016/j.ijfoodmicro.2016.11.005 [ Links ]
Hall TA.1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41:95-98. [ Links ]
Hartley JC. 1967. Laboratory culture of a tettigoniid, Homorocoryphus nitidulus vicinus (Wlk.) (Orthoptera). Bulletin of Entomological Research 57: 203-205. https://doi.org/10.1017/S0007485300049920 [ Links ]
Hinks CF, Erlandson MA. 1994. Rearing grasshoppers and locusts: review, rationale and update. Journal of Orthoptera Research 3: 1-10. https://doi.org/10.2307/3503403 [ Links ]
Inglis GD, Johnson DL, Goettel MS. 1996. Effects of temperature and thermoregulation on mycosis by Beauveria bassianain Grasshoppers. Biological Control 7(2): 131-139. https://doi.org/10.1006/bcon.1996.0076 [ Links ]
Insua JL, Llobet E, Moranta D, Pérez- Gutierrez C, Tomás A, Garmendia J, Bengoechea JA. 2013. Modeling Klebsiella pneumoniae pathogenesis by infection of the wax moth Galleria mellonella. Infection and Immunity 81(10): 3552-3565. https://doi.org/10.1128/IAI.00391-13 [ Links ]
Khan S, Guo L, Shi H, Mijit M, Qiu D. 2012. Bioassay and enzymatic comparison of six entomopathogenic fungal isolates for virulence or toxicity against green peach aphids Myzus persicae. African Journal of Biotechnology 11: 14193-14203. [ Links ]
Khaskheli MI, Khaskheli AJ, Jiskani MM, Chang X, Gong G, Poussio GB, Otho SA. 2019. The use of promising entomopathogenic fungi for eco-friendly management of Helicoverpa armigera Hubner in chickpea. International Journal of Environment, Agriculture and Biotechnology 4(2):705-712. https://doi.org/https://dx.doi.org/10.22161/ijeab [ Links ]
Kinyuru JN, Kenji GM, Muhoho SN, Ayieko M. 2011. Nutritional potential of longhorn grasshopper (Ruspolia differens) consumed in Siaya district, Kenya. Journal of Agriculture, Science and Technology 12: 32-46. [ Links ]
Kinyuru JN, Konyole SO, Kenji GM, Onyango CA, Owino VO, Owuor BO, Estambale BB, Friis H, Roos N. 2012. Identification of traditional foods with public health potential for complementary feeding in western Kenya. Journal of Food Research 1:148-158. https://doi.org/10.5539/jfr.v1n2p148 [ Links ]
Kumar S, Riffat S, Wagan MS. 2013. Pathogenic Application of Aspergillus species for the control of agricultural important grasshoppers. Journal of Biodiversity and Environmental Sciences 3(12): 223-229. [ Links ]
Labu S, Subramanian S, Khamis FM, Akite P, Kasangaki P, Chemurot M, Tanga CM, Ombura FLO, Egonyu JP. 2021. Microbial contaminants in wild harvested and traded edible long-horned grasshopper, Ruspolia differens (Orthoptera: Tettigoniidae) in Uganda. Journal of Insects as Food and Feed 7(7): 1131-1141. https://doi.org/10.3920/JIFF2020.0069 [ Links ]
Lacey L. 2012. Manual of techniques in invertebrate pathology. 2nd ed. Amsterdam, the Netherlands: Academic Press. [ Links ]
Lehtovaara VJ, Roininen H, Valtonen A. 2018. Optimal temperature for rearing the edible Ruspolia differens (Orthoptera: Tettigoniidae). Journal of Economic Entomology 111(6): 2652-2659. https://doi.org/10.1093/jee/toy234 [ Links ]
Lenth R, Lenth MR. 2018. Package 'lsmeans'. The American Statistician 34: 216-221. [ Links ]
Leonard A, Khamis FM, Egonyu JP, Kyamanywa S, Ekesi S, Tanga CM, Copeland RS, Subramanian S. 2020. Identification of edible shortand long-horned grasshoppers and their host plants in East Africa. Journal of Economic Entomology 113(5): 2150-2162. https://doi.org/10.1093/jee/toaa166 [ Links ]
Leonard A, Egonyu JP, Tanga CM, Kyamanywa S, Tonnang HZ, Azrag AG, Khamis FM, Ekesi S, Subramanian S. 2021. Predicting the current and future distribution of the edible long-horned grasshopper Ruspolia differens (Serville) using temperature-dependent phenology models. Journal of Thermal Biology 95:102786. https://doi.org/10.1016/j.jtherbio.2020.102786 [ Links ]
Malinga GM, Valtonen A, Lehtovaara VJ, Rutaro K, Opoke R, Nyeko P, Roininen H. 2018a. Diet acceptance and preference of the edible grasshopper Ruspolia differens (Orthoptera: Tettigoniidae). Applied Entomology and Zoology 53: 229-236. https://doi.org/10.1007/s13355-018-0550-3 [ Links ]
Malinga GM, Valtonen A, Lehtovaara VJ, Rutaro K, Opoke R, Nyeko P, Roininen H. 2018b. Mixed artificial diets enhance the developmental and reproductive performance of the edible grasshopper, Ruspolia differens (Orthoptera: Tettigoniidae). Applied Entomology and Zoology 53(2): 237-242. https://doi.org/10.1007/s13355-018-0548-x [ Links ]
Matojo ND, Yarro JG. 2013. Anatomic morphometrics of the "Senene" Tettigoniid Ruspolia differens Serville (Orthoptera: Conocephalidae) from North-West Tanzania. International Scholarly Research Notices 2013: 176342. https://doi.org/http://dx.doi.org/10.1155/2013/176342
Meshram PB, Verma P, Patel A, Verma RK. 2015. Entomopathogenic fungi of Albizia lebbeck seed borer, Bruchus bilineatopygus (Coleoptera: Bruchidae). International Journal of Current Research 7 (12), 24547-24551. [ Links ]
Miller SC, Campbell BC, Becnel J, Ehrman L. 1995. Bacterial entomopathogens from the Drosophila paulistorum semispecies complex. Journal of Invertebrate Pathology 65(2): 125-131. https://doi.org/10.1006/jipa.1995.1019 [ Links ]
Mukasa Y, Kyamanywa S, Sserumaga JP, Otim M, Tumuhaise V, Erbaugh M, Egonyu JP. 2019. An atoxigenic L-strain of Aspergillus flavus (Eurotiales: Trichocomaceae) is pathogenic to the coffee twig borer, Xylosandrus compactus (Coleoptera: Curculionidea: Scolytinae). Environmental Microbiology Reports 11(4): 508-517. https://doi.org/10.1111/1758-2229.12705 [ Links ]
Ng'ang'a J, Imathiu S, Fombong F, Ayieko M, Vanden Broeck J, Kinyuru J. 2019. Microbial quality of edible grasshoppers Ruspolia differens (Orthoptera: Tettigoniidae): From wild harvesting to fork in the Kagera Region, Tanzania. Journal of Food Safety 39(1): e12549. https://doi.org/10.1111/jfs.12549 [ Links ]
Okia CA, Odongo W, Nzabamwita P, Ndimubandi J, Nalika N, Nyeko P. 2017. Local knowledge and practices on use and management of edible insects in Lake Victoria basin, East Africa. Journal of Insects as Food and Feed 3: 83-93. https://doi.org/10.3920/JIFF2016.0051 [ Links ]
Opisa S, Du Plessis H, Akutse KS, Fiaboe KKM, Ekesi S. 2018. Effects of Entomopathogenic fungi and Bacillus thuringiensis-based biopesticides on Spoladea recurvalis (Lepidoptera: Crambidae). Journal of Applied Entomology 142(6): 617-626. https://doi.org/10.1111/jen.12512 [ Links ]
R Core Team. 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
De la Rosa W, Alatorre R, Barrera JF, Toriello C. 2000. Effect of Beauveria bassiana and Metarhizium anisopliae (Deuteromycetes) upon the coffee berry borer (Coleoptera: Scolytidae) under field conditions. Journal of Economic Entomology 93(5): 1409-1414. https://doi.org/10.1603/0022-0493-93.5.1409 [ Links ]
Shah PA, Kooyman C, Paraïso A. 1997. Surveys for fungal pathogens of locusts and grasshoppers in Africa and the Near East. The Memoirs of the Entomological Society of Canada 129: 27-35. https://doi.org/10.4039/entm129171027-1 [ Links ]
Shapiro-Ilan DI, Jackson M, Reilly CC, Hotchkiss MW. 2004. Effects of combining an entomopathogenic fungi or bacterium with entomopathogenic nematodes on mortality of Curculio caryae (Coleoptera: Curculionidae). Biological Control 30(1): 119-126. https://doi.org/10.1016/j.biocontrol.2003.09.014 [ Links ]
Sharma A, Thakur A, Kaur S, Pati PK. 2012. Effect of Alternaria alternata on the coccinellid pest Henosepilachna vigintioctopunctata and its implications for biological pest management. Biological Control 85(4): 513-518. https://doi.org/10.1007/s10340-012-0432-3 [ Links ]
Ssepuuya G, Mukisa IM, Nakimbugwe D. 2017. Nutritional composition, quality, and shelf stability of processed Ruspolia nitidula (edible grasshoppers). Food Science & Nutrition 5(1): 103-112. https://doi.org/10.1002/fsn3.369 [ Links ]
Ssepuuya G, Wynants E, Verreth C, Crauwels S, Lievens B, Claes J, Nakimbugwe D, Van Campenhout L. 2019. Microbial characterisation of the edible grasshopper Ruspolia differens in raw condition after wild-harvesting in Uganda. Food Microbiology 77: 106-117. https://doi.org/10.1016/j.fm.2018.09.005 [ Links ]
Suzuki MT, Giovannoni SJ. 1996. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Applied and Environmental Microbiology 62(2): 625-630. https://doi.org/10.1128/aem.62.2.625-630.1996 [ Links ]
Toledo AV, Virla E, Humber RA, Paradell SL, Lastra CL. 2006. First record of Clonostachys rosea (Ascomycota: Hypocreales) as an entomopathogenic fungus of Oncometopia tucumana and Sonesimia grossa (Hemiptera: Cicadellidae) in Argentina. Journal of Invertebrate Pathology 92(1): 7-10. https://doi.org/10.1016/j.jip.2005.10.005 [ Links ]
Tuininga AR, Miller JL, Morath SU, Daniels TJ, Falco RC, Marchese M, Sahabi S, Rosa D, Stafford KC . 2009. Isolation of entomopathogenic fungi from soils and Ixodes scapularis (Acari: Ixodidae) ticks: prevalence and methods. Journal of Medical Entomology 46(3): 557-565. https://doi.org/10.1603/033.046.0321 [ Links ]
Venables, W.N. & Dichmont, C.M. 2004. GLMs, GAMs and GLMMs: an overview of theory for applications in fisheries research. Fisheries Research 70(2-3), 319-337. https://doi.org/htts://dopi:10.1016/j.fishres.2004.08.011 [ Links ]
Wang C, Hu G, St. Leger RJ. 2005. Differential gene expression by Metarhizium anisopliae growing in root exudate and host (Manduca sexta) cuticle or hemolymph reveals mechanisms of physiological adaptation. Fungal Genetics and Biology 42(8): 704-718. [ Links ]
Yaman M, Ertürk Ö. 2016. Isolation, identification and insecticidal effects of entomopathogenic bacteria from the willow flea beetle, Crepidodera aurata (Coleoptera; Chrysomelidae). Progress in Plant Protection 56(2): 225-229. https://doi.org/10.14199/ppp-2016-037 [ Links ]
Youngjin P, Kim K, Kim Y. 2002. A pathogenic bacterium, Enterococcus faecalis, to the beet armyworm, Spodoptera exigua. Journal of Asia-Pacific Entomology 5(2): 221-225. https://doi.org/10.1016/S1226-8615(08)60156-9 [ Links ]
Zelazny B, Goettel MS, Keller B. 1997. The potential of bacteria for the microbial control of grasshoppers and locusts. The Memoirs of the Entomological Society of Canada 129: 147-156. https://doi.org/10.4039/entm129171147-1 [ Links ]
 Correspondence:
Correspondence:
Sevgan Subramanian
Email: ssubramania@icipe.org
Received: 02 July 2021
Accepted: 07 July 2022














