Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a15181
RESEARCH ARTICLE
Descriptions of a new Aedes species and subspecies of the subgenus Aedimorphus, from southwest Cameroon and updated key for the species of the "Domesticus group"
Cyril KowoI; Marie Paul Audrey MayiII; Antonio Paulo Gouveia de AlmeidaIII; David FonchaI; Mirabel EladI; Esack AndongmaI; Charlene DjomoIV; Jerome Fru-ChoI; Damian Nota AnongI; Ravinder SehgalVI; Anthony John CornelV
IDepartment of Microbiology and Parasitology, University of Buea, Buea, Cameroon
IIVector Borne Diseases Laboratory of the Research Unit of Biology and Applied Ecology (VBID-RUBAE), Department of Animal Biology, Faculty of Sciences of the University of Dschang, Dschang, Cameroon
IIIGlobal Health and Tropical Medicine (GHTM), Institute of Hygiene and Tropical Medicine (IHTM), NOVA University of Lisbon, Lisboa, Portugal
IVHigher Institute of Environmental Science, Yaounde, Cameroon
VMosquito Control Research Laboratory, Department of Entomology and Nematology and Vector Genetics Laboratory, Department of Pathology, Microbiology and Immunology, University of California at Davis, Parlier, USA
VIDepartment of Biology, San Francisco State University, San Francisco, USA
ABSTRACT
Male specimens of Aedes stenostylus Cornel, Kowo & Mayi sp. nov. and Aedes leptolabis ssp. talangayensis Cornel, Kowo & Mayi sp. nov. are described. They were collected mainly by sweep netting through forest floor vegetation in partially logged areas and in the surrounding pristine forest (Talangaye Forest) in the Nguti Subdivision in the south-west region of Cameroon. An updated key of the Aedimorphus "Domesticus group" species, based on the morphology of the male genitalia is provided.
Keywords: Aedes stenostylus sp. n, Aedes leptolabis ssp. talangayensis n.sp, Aedes ovazzai, Aedes tauffliebi
INTRODUCTION
Three species of Afrotropical Aedes subgenus Aedimorphus, namely Aedes domesticus Theobald, 1901, Aedes leptolabis Edwards, 1936 and Aedes longiseta Edwards, 1936, were described as morphologically homogeneous by Edwards (1941). A fourth species, Aedes ovazzai Hamon and Adam, 1959, was described by Hamon and Adam (1959) as also very similar to the three species recognised by Edwards (1941). For convenience, the four species were considered members of the "Domesticus group" (Hamon and Adam, 1959) that could only reliably be identified by differences in the male genitalia. Since then, a further three species considered members of the "Domesticus group" were described, and they are: Aedes tauffliebi Rickenbach and Ferrera, 1965 (Rickenbach and Ferrera, 1965), Aedes bambiotai Geoffroy, 1987 and Aedes bancoi Geoffroy, 1987 (Geoffroy, 1987). Aedes domesticus is in fact the species designated for the subgenus Aedimorphus (Harbach, 2013). "Domesticus group" species differs from other Aedimorphus by the combination of a reddish brown scutum bearing a pair of broad silver metallic scales on lateral scutal fossal areas, broad silver metallic scales on all lobes of scutellum, bare paratergites, sub medial white dorsal spot of silver metallic scales on the hind femur and dark tarsi.
In 2016 and 2017, mosquito sampling was conducted in the primary and secondary (partially logged) hardwood Talangaye forest located in the South-West Region of Cameroon. This area was selected as part of a study to measure the impacts of deforestation on the prevalence and dynamics of bird malaria in mosquitoes and birds (Tchoumbou et al. 2020). To measure bird malaria infectivity in mosquitoes, the relevant mosquitoes had to be identified correctly (Cornel et al. 2020). Externally both male and female member species of the "Domesticus group" look the same and can only be separated by careful examination of slide mounts of the male genitalia. All collected females were therefore identified as "Domesticus group" and preserved in alcohol for later avian parasite isolation. All males identified as "Domesticus group" were preserved individually in tubes with silica gel pellets to attempt to keep them dry for later pinning and genitalia examination. Three species of "Domesticus group" were identified as present in the forest: Aedes domesticus, Aedes ovazzai and Aedes leptolabis. However, Ae. leptolabis differed slightly from the description in Edwards (1941); so, based on this finding, we assigned it as a subspecies with name Ae. leptolabis ssp. talangayensis Cornel, Kowo & Mayi sp. nov. Furthermore, male genitalia from multiple specimens differed quite obviously from that of other currently described "Domesticus group" species and we, therefore, decided to describe these as a new species named Aedes stenostylus Cornel, Kowo & Mayi sp. nov.
In this paper, we comprehensively describe the males of Aedes leptolabis ssp. talangayensis sp. nov. and Aedes stenostylus sp. nov. and provide an updated key of species of the "Domesticus group" of the Aedimorphus subgenus of Aedes.
METHODS
Sampling area and notes about the environment
Sampling was carried out in the Talangaye Forest, Nguti Subdivision in the South-West Region of Cameroon within a 3-km radius of the GPS coordinates of 5.190397°, 9.3457790° E (Figure S1). The village nearest to our collecting sites is Manyamen. Before 2018, the area, which is hilly, was covered with lowland broad-leaf forest trees (dominated with Mahogony and Sapele hardwood trees) and bushes (Figures S2A and S2B). The relative humidity recorded was 97% RH during the wet season and temperatures ranged from 19-25 °C, measured using data loggers (HOBO-U23 Pro. Version 2, Onset Computer Corporation, Bourne, MA, U.S.A.) positioned in the shade 1 m above the ground. Within this area, a consortium of investors had plans to replace 700 km2 of pristine forest with plantations of palm oil and logging of large trees has in fact begun close to where we collected mosquitoes.
Mosquito capture
Mosquitoes were captured using sweep nets and net traps baited with chickens and pigeons (Columbus livia domestica Gmelin) and some adults were reared from immatures found in bamboo pots during January-February (late dry season), July (middle wet season) and October-November (early dry season) in 2016 before logging and then again in the same months in 2017 after logging had started.
Preservation and identification of mosquitoes
Collected mosquitoes were identified to genus and subgenus and, whenever possible, to species using a dissecting microscope. Females were identified as "Domesticus group". Most males were preserved individually in a 1-ml tube with silica gel, and some were pin-mounted in the field as described by Cornel et al. (2020). Digital images of the adult morphological characteristics and genitalia were taken using an OMAX A3518OU attached to a Nikon SMZ800 dissecting microscope and an Amscope MU2003-Bl attached to a Nikon E600 compound microscope, respectively, with phase contrast optics. Images were processed using Amscope Capture software version 3.7 (Amscope, Irvine, CA, U,S,A,). Mosquito anatomical terminology follows that recommended in Harbach (2013) and Harbach & Knight (1980, 1982).
TAXONOMIC TREATMENT
Aedes leptolabis ssp. talangayensis Cornel, Kowo & Mayi sp. nov.
Male. Vestiture closely resembles all other members of "Domesticus group". Male differs from typical Ae. leptolabis in having differences in the numbers and positions of setae on the basal medial lobe (claspette) of the male genitalia, and a longer white apical spot on the hind tibia. Named subspecies talangayensis in reference to location.
Head. White to cream-coloured narrow decumbent scales and black erect forked setae on the vertex (Figure 1B (a)), broad black decumbent scales in interocular space (Figure 1B (b)) and adjoining eyes between vertex and postgena (Figures 1B, C and D (c)), pair of broad silver patches adjoining eyes not meeting in the middle (Figures 1B and C (d)), postgena covered with creamy-coloured scales (Figure 1C (e)), a patch of decumbent broad silver scales on upper postgena adjoining eyes below black scales patch (Figure 1C and D (ƒ)). Antenna shorter than proboscis with basal 12 flagellomeres bearing numerous highly light reflective flagellar setae directed mainly dorsally and ventrally, apical two flagellomeres lack flagellar whorls and are sparsely covered with very short setae and two longer setae basally (Figure 1A). Maxillary palpus (2028 μm) is slightly longer than the proboscis (1845 μm) (Figure 1A). The proboscis and maxillary palpus are dark although the ventral surface of the maxillary palpus may appear pale because of descaling especially in older specimens. Final two maxillary palpus segments more setose. Pedicel light brown and bare.

Thorax. Exoskeleton of scutum reddish brown with acrostichal and dorsocentral setae sparsely but quite evenly covered with narrow dark brown scales except for a conspicuous round patch (approximately 244 χ 156 μm diameter) on each fossal area of broad flat silver scales; scutellum completely clothed with silver broad scales (Figure 1A). Exoskeleton of pleuron reddish brown (Figure 2A). Paratergite bare. No scales on the postpronotum but a row of dark setae along the dorsal edge, antepronotum bearing setae only. On the rest of the pleuron proepisternal (PScU), upper mesokatepisternal (MScU), lower mesokatepisternal (MScL) and upper mesepimeral (UMSc) patches of silver broad scales (Figure 2A). No prealar scales but numerous setae present. Post spiracular setae present. Haltere stalk beige and knob black. Mesopostnotum bare.
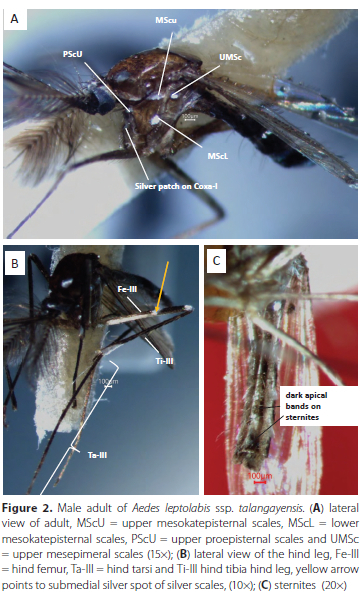
Wings. Dark scaled with a small silver spot of scales at the base of the costa (Figure 1A). Length: 2438 μm. Fore legs. Upper anterior VV of coxa with a patch of silver broad scales (Figure 2A) and rest of coxa covered sparsely with narrow dark scales and dark setae. Femur has a very small apical silvery-white spot. Tibia with dorso-apical silvery-white spot (251 μm). Tarsi dark. Fore ungues unequal, larger unguis hooked and smaller unguis simple. Lengths of leg segments: Fe-I = 1489 μm Ti-I = 1525 μm Ta-I1 = 936 μm Ta-I2 = 326 μm Ta-I3 = 166 μm Ta-I4 = 82 μm Ta-I5 = 59 μm
Mid legs. Coxa covered sparsely with narrow dark scales and a row of dark setae. Femur with a very small dorsal apical silvery-white spot. Tibia all dark. Tarsi dark. Both mid ungues are hooked and unequal. Lengths of leg segments: Fe-II = 1542 μm, Ti-II = 1774 μm Ta-II1 = 1178 μm Ta-II2 = 525 μm Ta-II3 = 188.81 μm Ta-II4 = 91 μm, Ta-II5 = 110 μm
Hind legs. Coxa covered sparsely with narrow dark scales and row of dark setae. Femur with apical silvery-white spot 129 μm in length; preapical spot of silvery white scales 235 μm in length (yellow arrow in Figure 2B). Basal half of lateral ventral sides of femur cream to coloured (Figure 2B). Tibial silver anterior apical spot (354 μπι) about one-fifth of tibial length (Figure 2B). Hind ungues small, equal and simple. Lengths of leg segments: Fe-III = 1651 μm, Ti-III = 1868 μm, Ta-III1 = 1487 μm, Ta-III2 = 909.73 μm, Ta-III3 = 598 μm, Ta-III4 = 358 μm, Ta-III5 = 323 μm.
Abdomen. Males have alrnost complete basal silvery-white tergal bands with obvious lateral basal broad silver patches of scales. Sternites with darker apical bands that are not obvious in all specimens (Figure 2C).
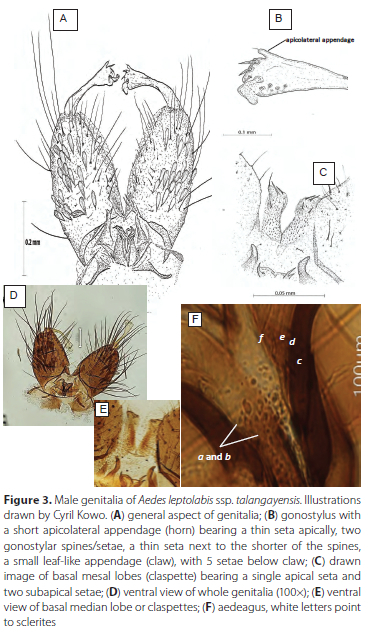
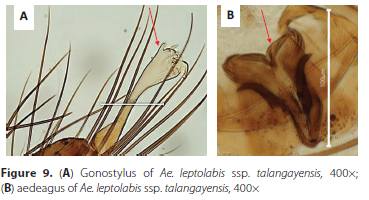
Male genitalia. Resembles Aedes leptolabis as described by Edwards (1941). Tergum IX is slightly indented in the middle creating appearance of two lobes with 5-7 long fine long setae positioned generally in two rows on each lobe. Gonostylus with short apicolateral appendage bearing apically a thin seta, 2 spines, one thin spine and a small leaf-like appendage (gonostylar claw) with five thin setae below claw (Figure 3A, B and D). Basal mesal lobe is covered with many fine setae and bears one apical and two more ventrally positioned quite stout subapical setae (Figure 3A, C and E) and two to three sub-medial setae. Gonocoxite length: 295.63 μm, Gonostylus (length = 189.51 μm, maximum width at club = 43.07 μm and width at narrowest point = 9.3 μm). Aedeagus about 85 μm in length consisting of on each side, ventrally two dark brown sclerites (Figure 3F (a and b)) and below a plate of four loosely connected light brown sclerites with the apical two sclerites much longer than the lower two (Figure 3F, (c to ƒ)), most basal denticle distinctly the darkest. The aedeagus sclerites are loosely connected and their relative positions often get distorted in slide mounts.
Females, pupae, larvae and eggs. Unknown.
Specimens examined
Holotype male (Bohart Museum type #1939), with genitalia on microscope slide that was reared from an egg laid in a bamboo pot that was attached 0.915 m above ground to the stem of a tree in Cameroon, Southwest Region, Nguti Subdivision, Talangaye Forest (5.175428° N, 9.455888° E) with the following label: Talangaye forest, South West Region, Cameroon, reared from bamboo pot, Acc. # CAM 85t 29/x/2016, adult male, genitalia mounted, Det. A. J. Cornel. A further 16 males of the same species name were collected resting in forest floor vegetation within 3 km radius from holotype and have Acc. #s CAM202hk 12/vii/17, CAM132h 4/ii/17, CAM132nb 25/i/17, CAM132n1 30/i/17, CAM132m 7/ii/17, CAM202ga 13/vii/17, CAM202hl 12/ vii/17, CAM202hg 12/vii/17, CAM202hb 12/vii/17, CAM202hp 12/vii/17, CAM202ih 16/vii/17, CAM202ic 21/vii/17, CAM132mb 26/i/17, CAM132r1 26/i/17, CAM202gc 13/vii/17, CAM132if 28/i/17 (dates of collection correspond to date on Acc. #s). Slide mounts of the genitalia have the same labels as the corresponding male carcass. The holotype and other specimens are deposited in the Bohart Museum, University of California at Davis, California, U.S.A.
Bionomics and Remarks. The holotype male was reared from an egg that was laid in a bamboo pot in a primary forest (when the forest was still intact) in the southwest region of Cameroon during the early dry season. Immatures of all other currently described members of the "Domesticus group" were found in forest floor ground pools (Hopkins 1952; Hamon and Adam 1959; Geoffroy 1987). Nothing more is known about the ecology of this new subspecies. No isolations of avian malaria were made from any of the pools of "Domesticus group" females.
Specimens were mainly collected by sweep netting and one specimen, the holotype, was reared from an immature in a bamboo pot. Specimens were collected in October 2016 within January-February 2017, and July 2017 both before and after large hardwood tree logging took place in Talangaye forest in Cameroon and the distribution beyond this location is not known. Variations in adult male morphology of Ae. leptolabis has been observed and is considered a polytypic species by Ribeiro et al. (1984). According to Edwards (1941) the length of the white spot on the hind tibia is smaller (¼ length of tibia) in Ae. leptolabis than in other "Domesticus" groups species (½ length of tibia). In our opinion, the length of the hind tibia white apical spot cannot be reliably used to identify Ae leptolabis because these spots were longer in specimens collected in Kenya (¼ as long as the tibia) (van Someren et al. 1955) and were about ½ as long as the hind tibia in Talangaye forest males.
In a specimen collected in Angola (Luachimo River bank, Carisso Park, Dundo, Angola, 23.XII.1960, New Jersey trap) the white spots length is short and is about ¼ the length of the hind tibia. Male specimens from Kenya had no complete basal silver bands on tergites (van Someren et al. 1955) whereas those from Talangaye forest that we collected and those examined by Edwards (1941) from the west and central Africa all had complete bands. Van Someren et al. (1955) described specimens from Kenya as having fore- and mid-legs with large apical white spots on tibiae but none on femora, while specimens from Talangaye have small dorsal apical white spots on fore- and mid-femora. The male from Angola has dorsal apical white spots on mid and hind femora but not on fore-femur. Van Someren et al. (1955) noted that the basal mesal lobe of the gonocoxite of male genitalia on specimens from Kenya were covered with short fine hairs with a long seta positioned in the inside middle region of the lobe and two short spines apically.
In Talangaye specimens, the basal mesal lobe (claspette) was also covered with short fine setae and had two to three longer setae about in the middle of the lobe and a single distinctly stouter apical setae and two more slightly less stout ventrally positioned subapical setae. Edwards (1941) described the medial lobe as having a "few short hairs" in specimens he examined. In the male from Angola the basal mesal lobe was covered in fine setae and had a single less stout apical seta, two stouter sub apical seta and two fine long setae ventrally in the middle of the lobe.
Aedes (Aedimorphus) stenostylus Cornel, Kowo & Mayi sp. nov.
Unfortunately, all the specimens of this species are in poor condition due to fungal growth on them which occurred while we were in the forest which at the time rained heavily every day for several hours, creating a very wet and humid climate. Only after we dissected and examined the genitalia did we discover differences in the gonostyle, median lobe (claspette) and aedeagus from the other "Domesticus group" members to warrant consideration of finding a new species. This species is named stenostylus in reference to the narrow gonostyle that lacks an apicodorsal extension.
No differences in external morphological features were found between Ae. stenostylus and Ae. leptolabis ssp. talangayensis. The morphological features described above for Ae. leptolabis ssp. talangayensis male applies to Ae. stenostylus male as well. The only differences were found in male genitalia structure which is described below.
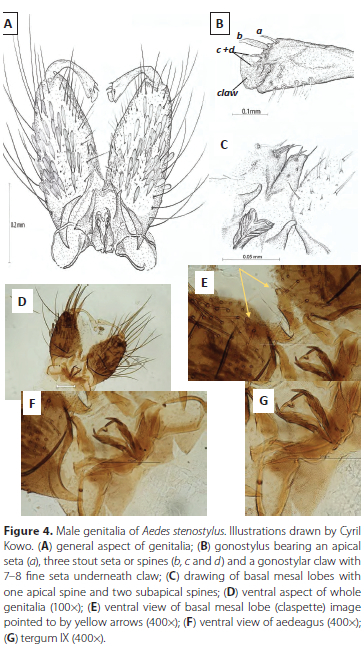
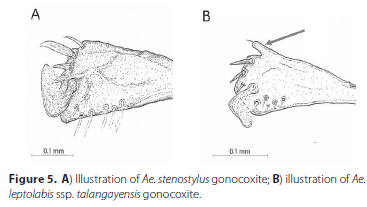
Male Genitalia. Tergum IX is slightly indented in the middle creating a lobed appearance with one lobe bearing 5 and the other 6 long fine setae subapically (Figure 4G). Gonocoxite length: 290.95 μπι (Figure 4D). Gonostylus (Length = 199.31 μm, width at narrowest part = 10.16 μm and width at the broadest part (apex) = 38.34 μm) lacks horn or apicolateral extension whereas this is present in all the other species of the "Domesticus group" (Figure 4A and B). Gonostyle apex from the outside edge to the gonostylar claw has a fine seta (Figure 4B (a)), stout seta or spine (Figure 4B (b)) and two short setae or spines (Figure 4B (c +d)) and 7-8 short fine seta below the. The basal mesal lobe (claspette) bears 1 apical and 2 more stout sub apical setae, and 1-3 sub medial setae (Figures 4C and E). The aedeagus comprises four shorter denticles or sclerites followed by 2 longer less rigid denticles (Figure 4F).
Females, pupa, larva and eggs. Morphology unknown.
Specimens examined
Holotype male (Bohart Museum type #1938), with genitalia on microscope slide that was captured resting in forest floor vegetation using a sweep net in Cameroon, Southwest Region, Nguti Subdivision, Talangaye Forest (5.19039°N, 9.345779°E) with the following label: Talangaye forest, Southwest Region, Cameroon, reared from bamboo pot, Acc. # CAM 202hm 12/vii/ 2017, adult male, genitalia mounted, Det. AJ Cornel. A further 14 males of the same species name were collected resting in forest floor vegetation within 3 km radius from holotype and have Acc. #s CAM 202hn 12/vii/17, CAM 202gb 13/vii/17, CAM 202ho 12/ vii/17, CAM 202hf 12/vii/17, CAM 202hd 12/vii/17, CAM 202ha 12/vii/17, CAM 202ga 9/vii/17, CAM 202gd 9/vii/17, CAM 202gc 9/vii/17, CAM 202ma 17/vii/17, CAM 202ib 21/vii/17, CAM 202if 16/vii/17CAM213e 13/vii/17, and 202e 20/vii/17 (dates of collection correspond to date on Acc. #s).
Bionomics and Remarks. All specimens of this new species were all collected in the wet season during July 2017) in Talangaye forest in Cameroon and their distribution beyond this location is not known. Nothing more is known about the ecology of this species.
Identification key to the males of the "Domesticus group" of Aedimorphus based on genitalia
Characters used in the keys for those species that were not collected by us are taken from published descriptions and drawings. Arrows in the figures in the key point to features mentioned in the couplets.
2. Basal mesal lobe with single very long apical seta about as long as lobe itself..................................Ae. longiseta Edwards - Basal mesal lobe has a short apical seta that is much shorter than lobe itself (Figure 6).......................................3
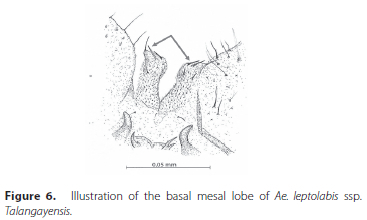
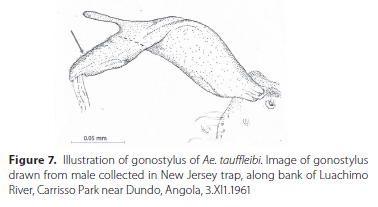
3. Gonostylus apex is covered in fine setae and appears as an elongated triangle extending outwards from the apicodorsdal appendage, fine setae along the upper edge of triangular extension and gonostylar claw at end of elongated extension (Figure 7)....................Ae. tauffliebi Rickenbach and Hamon - Apex of gonostylus otherwise more club-shaped...........4
4. Club on apex of gonostylus very enlarged (more than 5 times the width of gonostylus base)..................................................5 - Club on apex of gonostylus not more than 5 times width of gonostylus base .............................................................6
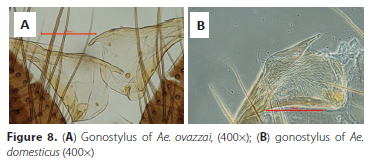
5. Club of gonostylus has no fine setae or villi (Figure 8A, arrow)..........................................Ae. ovazzai Hamon & Adam - Club of gonostylus covered with fine setae (Figure 8B, arrow)................................................Ae. domesticus Theobald
6. Apex of gonostylus with row of 7 setae/spines between apicodorsal arm and gonostylar claw....................................... .......................................................Ae. bambiotai Geoffroy - Apex of gonostylus with 3 or 4 setae/spines between apicodorsal arm and gonostylar claw............................7
7. Apicodorsal extension or horn long (length extending to almost level with tips of apical setae/spines, 3 setae /spines between apicodorsal extension and gonostylar claw; 5-6 short denticles on each of aedeagus......Ae. bancoi Geoffroy - Apicodorsal extension or horn shorter (length not extending to beyond VV length of apical setae/spines between apicodorsal extension and gonostylar claw (Figure 9A, arrow); apical two sclerites on each side of aedeagus much longer than other sclerites (Figure 9B, arrow) ....................... Ae. leptolabis Edwards, Ae. leptolabis ssp. talangayensis Cornel, Kowo, & Mayi
ACKNOWLEDGEMENTS
We thank funders of the USAID PEER grant (4-360 grant awarded AID 0AA-11-00012) which paid salaries and field trip costs for Cameroon students and University faculty to collect mosquitoes in the field. We also thank the National Geographic Society (grant # 983616) for funding AJC to travel to assist in the collection of mosquito and bird blood samples. We are also grateful for the financial assistance awarded to Marie Paul Audrey Mayi from the National Geographic Society (grant # WW-117ER-17) that paid for her to travel to the field sites and to purchase field collecting equipment, mosquito preservation reagents and consumables. Marie Paul Audrey Mayi also received financial assistance from the National Geographic Society (''Support for Women and Dependent Care-1399) for a visit to AJC's laboratory to assist in the examination of specimens and preparation of this manuscript. We equally wish to thank Dr Kevin Njabo for facilitating the training of students on the necessary quantitative analytical skills. Ethical approval for the use of chickens and pigeons as mosquito baits for the net traps was obtained from the Animal Care and Use SubCommittee, Research Ethics Committee, University of Buea, IACUC Protocol number UB-AP_2015-004.
AUTHORS' CONTRIBUTIONS
C Kowo: Data curation, investigation, writing-original manuscript, writing-review, and editing.
MPA Mayi: Data curation, investigation, writing-original manuscript, writing-review and editing, resources.
APG de Almeida: data curation, visualisation, resources, writing-review and editing.
D Foncha: Data curation and investigation, writing-review and editing.
M Elad: Data curation and investigation, writing-review and editing.
E Andongma: Data curation and investigation, writing-review and editing.
C Djomo: Data curation and investigation, writing-review and editing.
J Fru-Cho: Supervision, writing-review and editing.
DN Anong: Conceptualisation, funding acquisition, project administration, supervision, and writing-review and editing.
R Sehgal: Conceptualisation, methodology, funding acquisition, data curation, investigation, formal analysis, resources, supervision, visualisation, validation, writing-review and editing.
A Cornel: Conceptualisation, methodology, data curation, investigation, formal analysis, resources, supervision, visualisation, validation, writing original manuscript, and writing review and editing.
ORCID IDs
Cyril Kowo - https://orcid.org/0000-0002-1880-1733
Marie Paul Audrey Mayi - https://orcid.org/0000-0003-2735-8232
Antonio Paulo Gouveia de Almeida - https://orcid.org/0000-0003-0751-4488
David Foncha - https://orcid.org/0000-0002-5476-1978
Mirabel Elad - https://orcid.org/0000-0001-9090-1459
Esack Andongma - https://orcid.org/0000-0002-3087-5700
Ravinder Sehgal - https://orcid.org/0000-0002-5255-4641
Anthony John Cornel - https://orcid.org/0000-0003-2735-8232
REFERENCES
Cornel AJ, Mayi MPA, Kowo C, Foncha D, Andongma E, Anong DN, Elad M, Djomo C, Tchuinkam T, Brisco KK, et al. 2020, New species of Culex (Culiciomyia) (Diptera: Culicidae) from Talangaye Forest in Cameroon and descriptions and identification keys for males of the Afrotropical species of the subgenus. Zootaxa 4858(4):451-506. https://doi.org/10.11646/zootaxa.4858.4.1 [ Links ]
Edwards FW. 1936. New African culicine mosquitoes (Diptera, Culicidae). Proceedings of the Royal Entomological Society of London Series B Taxonomy 5(3):49-55. https://doi.org/10.1111/j.1365-3113.1936.tb00593.x [ Links ]
Edwards FW. 1941. Mosquitoes of the Ethiopian Region III.-Culicine adults and pupae. London: British Museum (Natural History). [ Links ]
Geoffroy B. 1987. The Aedes (Aedimorphus) Domesticus Group (Diptera, Culicidae). I. New species, descriptions of Aedes bambiotai and Aedes bancoi. Mosquito Systematics 19:100-110. [ Links ]
Hamon J, Adam JP. 1959. Description de deux nouveaux Aedes de Cote d'Ivoire appurtenant au sous-genre Aedimorphus: Ae. ovazzai sp.n. et Ae. rickenbachi sp.n. Bulletin de la Société de pathologie exotique 52:147-154. [ Links ]
Harbach RE. 2013. Mosquito Taxonomic Inventory. https://mosquito-taxonomic-inventory.myspecies.info/ [accessed 26 September 2022].
Harbach RE, Knight KL. 1980. Taxonomists' Glossary of Mosquito Anatomy. Marlton (New Jersey): Plexus Publishing. [ Links ]
Harbach RE, Knight KL. 1982. Corrections and additions to "Taxonomists' glossary of mosquito anatomy." Mosquito Systematics. 13: 201-217. [ Links ]
Hopkins GHE. 1952. Mosquitoes of the Ethiopian Region-I. -Larval bionomics of mosquitoes and taxonomy of culicine larvae. 2nd edition. London: British Museum (Natural History). [ Links ]
Rickenbach A, Ferrara L. 1965. Description de deux nouveaus Aedes du Cameroun appurtenant au Sous genre Aedimorphus (Diptera, Culicidae). Bulletin de la Société de pathologie exotique 58:24-29. [ Links ]
Ribeiro H, da Cunha Ramos H, de Barros Machado A. 1982. Research on the mosquitoes of Angola (Insecta, Diptera, Culicidae) XIII -Twelve new records from the Lunda Province. Anais do Instituto de Higiene e Medicina Tropical 8:125-130. [ Links ]
Tchoumbou MA, Mayi MPA, Malange ENF, Foncha FD, Kowo C, Fru-cho J, Tchuinkam T, Awah-Ndukum J, Dorazio R, Nota Anong D, et al. 2020. Effect of deforestation on the prevalence of avian haemosporidian parasites and mosquito abundance in a tropical rainforest of Cameroon. International Journal of Parasitology 50(1):63-73. https://doi.org/10.1016/j.ijpara.2019.10.006 [ Links ]
Theobald FV. 1901. A Monograph of the Culicidae, or Mosquitoes. Volume I. London: British Museum (Natural History). [ Links ]
van Someren ECC, Teesdale C, Furlong M. 1955. The mosquitos [sic] of the Kenya Coast; records of occurrence, behaviour and habitat. Bulletin of Entomological Research 46(3):463-493. https://doi.org/10.1017/S0007485300039481 [ Links ]
 Correspondence:
Correspondence:
Cyril Kowo
Email: kowocyril@gmail.com
Received: 17 November 2022
Accepted:31 January 2023














