Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a14183
RESEARCH ARTICLE
Entomopathogenic fungi associated with cultivated honeybush, Cyclopia spp., in South Africa and their pathogenicity towards a leafhopper pest, Molopopterus sp. (Hemiptera: Cicadellidae)
TG Mushore; CA Coombes; M Hill
Centre for Biological Control, Department of Zoology and Entomology, Rhodes University, Makhanda, South Africa
ABSTRACT
The southern and eastern parts of the African Fynbos region favour the production of honeybush tea. Honeybush biomass and extracts are used to prepare a beverage both locally and internationally, mainly as herbal tea with health benefits. Honeybush tea is mostly grown organically requiring natural control measures for pests and diseases. The leafhopper, Molopopterus sp., is one of the most important pests of cultivated honeybush in South Africa, as its feeding compromises the quality and quantity of the yield through leaf discolouration and reduction of the photosynthetic area. Local entomopathogenic fungi (EPF) can provide a pool of potential biocontrol agents for this pest. Therefore, a total of 98 soil samples were collected from organically grown honeybush fields and vegetation surrounding the honeybush fields in the Western Cape province of South Africa. Entomopathogenic fungi were isolated using the insect bait method and were characterised using molecular techniques. Twenty fungal isolates of Metarhizium anisopliae and Fusarium oxysporum were recovered from soil samples, of which 70% were from honeybush fields and 30% were from surrounding vegetation. Fusarium oxysporum isolates comprised 20% of the recovered isolates; M. anisopliae the remainder. Laboratory bioassays of the recovered isolates against adults and nymphs of the leafhopper, showed that F. oxysporum isolates caused 10-45% mortality and M. anisopliae isolates 30-80% mortality. Metarhizium anisopliae isolates J S1, KF S3, KF S11, KF S13, LSI and LS2 were the most virulent and induced over 60% mortality in both nymphs and adults at a concentration of 1 χ 107 conidia/ml.
Keywords: insect management, microbial control, pest management
INTRODUCTION
Honeybush, Cyclopia spp. Vent (Fabaceae), is a papilionaceous genus endemic to South Africa, used to produce a unique herbal tea with a pleasant honey-like taste and flavour (Schutte 1995; McGregor 2018). Honeybush tea has gained global recognition due to its enjoyable flavour and, like other herbal teas, it does not contain caffeine (McGregor 2018). Currently, consumer demand for honeybush products far outweigh the supply. This growing demand for honeybush tea, both locally and globally, has resulted in commercialisation of the industry and a shift from wild harvesting to dedicated planting (Joubert et al. 2011; Slabbert et al. 2019). The shift in cropping can cause changes in insect populations and possible pest outbreaks (Andow 1983). The honeybush tea industry is a certified organic industry (Department of Agriculture, Forestry and Fisheries 2014) and thus has zero tolerance towards the use of synthetic pesticides, which dictates pest control options.
The leafhopper, Molopopterus sp. Jacobi (Hemiptera: Cicadellidae), is a sap-sucking pest of agricultural importance (Hatting 2017). Both adults and nymphs suck plant sap mainly from the underside of the leaf, causing phytotoxic symptoms termed "hopper burn," which can result in complete desiccation. Hopper burn results from a dynamic feeding interaction between insect feeding stimuli and a complex plant response (Habibi et al. 2001). The leafhopper Molopopterus theae Theron (Hemiptera: Cicadellidae) is one of the major pests associated with Rooibos Aspalathus linearis (Burm.f.) R. Dahlgren (Fabaceae), a close relative of honeybush. The pest causes chlorosis, and under high leafhopper populations plants have a scorched appearance. As a result, there is impaired plant growth and, in extreme cases, mortality, which is exacerbated by moisture stress especially during seasonal summer drought from November to February (Hatting 2017). Entomopathogenic fungi (EPF) have been explored as a chemical alternative for control of the leafhopper associated with Rooibos (Hatting 2017). Metcalf et al. (2018) called for pre-emptive control measures due to the damage potential of Molopopterus sp. in Honeybush, which may result in considerable damage to Honeybush compromising yield quality and quantity.
The use of EPF as mycoinsecticides plays a key role in sustainable pest management programmes as almost all insects are vulnerable to fungal disease (Mochi et al. 2005). They have garnered significant interest in research and used as mycoinsecticides because they can be administered in ways similar to those used for other synthetic pesticides (Zimmermann 2007; Hussain et al. 2014). They are especially ideal in that they are eco-friendly, have a broad host range and they pose little threat to humans with minimal residues (Bidochka et al. 2001; Barra et al. 2012). Entomopathogenic fungi are considered advantageous in the control of tea pests as they are cost-effective, may increase yield as potential endophytes, and have minimal side effects on natural enemies (Hussain et al. 2014; Wang et al. 2018). They are used for the management of tea pests on their own or in combination with synthetic insecticides (Hazarika & Puzari 2001). In Brazil, EPF have been used successfully for the management of both nymphal and adult stages of spittlebugs in sugarcane (Mascarin et al. 2019). In the case of leafhoppers, they are an important biocontrol alternative since their pathogenesis process does not require ingestion (Thomas & Read 2007; Khan et al. 2012).
The objectives of this study were therefore to: (i) isolate and identify indigenous isolates of EPF from soil samples obtained from honeybush agro-ecosystems (cultivated and natural refugia) and; (ii) investigate the pathogenicity of the isolated EPF against both the adults and nymphs of Molopopterus sp. under laboratory conditions.
MATERIALS AND METHODS
Soil sampling
Soil samples were collected from three Honeybush farms in the Western Cape, where it is predominantly grown in South Africa; in Plettenberg Bay (33°55'55"S, 23°30'31"E), Sedgefield (33°58'36"S, 22°51'50"E) and George (33°56'09"S, 22°12'53"E). Soil samples were collected from honeybush fields and surrounding refugia. A total of 98 rhizospheric soil samples were collected using the sampling procedure described by Goble et al. (2010). Twelve soil samples, roughly 12 m apart, were collected per field along two intersecting transects. Twelve samples were also collected on each farm from the natural refugia within a 2 km radius of the field, with each sampling point 18 m apart. A cylindrical soil auger (7 cm χ 14 cm) with 538 cm3 volume was used to collect soil to a depth of 15 cm after the surface litter was removed. The soil auger was wiped with 70% ethanol between each sampling point to limit cross-contamination. Samples were collected at the beginning of April 2019. The soil samples were placed in individually labelled plastic ziplock bags, stored at 4 °C and baited within two months of the sampling period.
Isolation of entomopathogenic fungi from soil samples
Entomopathogenic fungi were isolated using the modified 'insect bait method' as described by Meyling (2007) and Meyling & Eilenberg (2006) with Tenebrio molitor L. (Coleoptera: Tenebrionidae) late instars used as the bait species. Tenebrio molitor were obtained from cultures kept at Rhodes University reared in plastic containers on an artificial diet comprised of a mixture of oats and wheat bran as protein sources. Potatoes were supplied as a carbohydrate source and to provide moisture. Soil samples were sieved through a sterile metal sieve with a mesh size of 4 mm to remove any unwanted material and make the soil loose to facilitate movement of bait insect within the soil samples, after which they were thoroughly mixed. If the soil was dry, the samples were lightly moistened with sterile distilled water to ensure enough moisture was present to promote fungal infection during baiting. Two 200 ml portions were measured and transferred to 400 ml transparent plastic containers, previously sterilised with 70% ethanol. Ten late instar larvae of T. molitor per soil sample were added and the containers sealed.
No heat treatment was applied to the bait insects since T. molitor do not create webbing. Soil samples were then incubated in complete darkness at ± 25 °C. Containers were inverted daily for the first week to encourage larval movement throughout the soil profile. The soil samples were lightly moistened with distilled water if they became too dry and were checked for dead insects every three days, a week after baiting, for a period of 21 days. All dead larvae were surface sterilised by first washing them in 70% alcohol then 1% sodium hypochlorite for 30 seconds, before being quickly rinsed three times with sterilised distilled water to prevent growth of opportunistic external saprophytic fungi (Oliver et al. 2011). After surface sterilisation, they were placed in an incubation chamber (sterile 90 mm Petri dish lined with moist filter paper) and kept in a controlled environment room at 25 °C, 12:12 light/dark regime and monitored for fungal outgrowth. Sporulating larvae and pupae were placed on Sabouraud Dextrose Agar (SDA) supplemented with 50 mg/l of chloramphenicol for fungal isolation. Following sufficient growth on the media, conidia were removed from the outgrowth surrounding the insect cadavers and diluted in Tween 20 (1:100) before being spread on prepared media, where they were incubated at room temperature. After three to four days, individual colonies were then aseptically removed and transferred to new plates to obtain pure cultures. The plates were grown for two weeks and later stored in a fridge at 4 °C prior to bioassays being conducted (Meyling 2007).
Molecular identification of recovered fungal isolates
Molecular characterisation was done for all the recovered fungal isolates. DNA was isolated following a modified protocol by Marzachi et al. (1998). The fungal samples were extracted from the agar plates using a 1000 μl sterile pipette tip, which was melted with a lighter, creating a ball that was then used to macerate the fungi. The samples were macerated in 200 μl sterile distilled water in a 2 ml microcentrifuge tube, after which 180 μl of ATL buffer (Qiagen) and 15 μl of proteinase K were added. The samples were then vortex mixed for 30 seconds prior to being placed in a heat block overnight at 56 °C. The samples were then centrifuged at 13 000 rpm for 5 minutes, after which the supernatant was removed and transferred to a new Eppendorf tube where 65 μl of 5M sodium chloride was added to the supernatant. The samples were then vortexed for 30 seconds and again centrifuged for 5 minutes at 13 000 rpm after which the supernatant was removed and transferred to a new Eppendorf tube, where 150 μl of cooled isopropanol was added. The samples were then placed in the freezer overnight and then centrifuged again at 13 000 rpm for 5 minutes. The supernatant was removed, and the DNA pellet was kept. Cooled 70% ethanol (250 μl) was added to the pellet and centrifuged at 13 000 rpm for 5 minutes after which the ethanol was removed. The Eppendorf tube was placed overnight on a heat block at 50 °C to allow any excess ethanol to evaporate. The DNA pellet was then dissolved in 20-40 μl of AE buffer (Qiagen).
A nanodrop spectrophotometer (Thermo Scientific*) was used to determine DNA concentration. Universal fungal primers were used to amplify the internal transcriber spacer region using the primers ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3'). Agarose Gel Electrophoresis (AGE) was used to analyse the PCR products to confirm whether amplification of the ITS region was successful. The following PCR cycling parameters were used: initial denaturation step of 94 °C for 5 minutes, followed by 35 cycles of 94 °C for 30 seconds, 52 °C for 45 seconds, 72 °C for 1 minute and finalised by one cycle at 72 °C for 5 minutes. The samples were run on 1% AGE (Marzachi et al. 1998). The samples were then sent to Inqaba Biotechnical Industries Pty Ltd (South Africa) for sequencing. Chromatograms were analysed in Finch TV v1.4.0 (Geospiza, Inc., U.S.A.) and samples were identified using the BOLD and GenBank database. Sequences were aligned using Chromas (v 2.6.6) and analysed using the maximum likelihood method in MEGA-X (v 10.2.2).
Fungal pathogenicity bioassays against Molopopterus sp. Preparation of conidia suspension
Fungal isolates were cultured for two weeks on SDA before conidia suspensions were prepared. The plates were then flooded with 20 ml of sterile 0.05% Tween 20 solution. A sterile 1000 μl pipette tip was used to gently scrape the surface of the media to dislodge the conidia before transferral into a sterile glass bottle. The suspension was then vortexed for 5 minutes to ensure homogenisation before determining the concentration of the conidia suspension. The concentration of the stock conidia suspension was determined using a Neubauer haemocytometer (Marienfeld, Germany) before dilution. Two counts were made per suspension and the average thereof used to determine the concentration of the stock suspension. For each isolate, a 1 χ 107 conidia/ml suspension was then prepared for use in the bioassays.
Conidia viability was assessed for each conidial suspension using the method described by Meyling (2007). A 1 χ 105 conidia/ ml suspension (100 μl) was aseptically spread onto freshly prepared SDA plates. Three replicates were made for each fungal isolate. The plates were wrapped with foil paper and incubated for 24 hours at 26 °C. A total number of 100 conidia per plate were counted under a light microscope (Olympus CX2LED) at low magnification. Germinating conidia were differentiated from non-germinating conidia by the presence of a visible germ tube (Meyling 2007). The average germination percentage was calculated.
Plant material and insect source
Adult Molopopterus sp. were collected from Cyclopia longifolia Vogel (Fabaceae) plants cultivated at Kurland using an active beating method. Infested plants were actively beaten by hand to dislodge the insects while a sweep net (diameter 32 cm χ 55 cm length) was positioned below to catch the insects. An electric pooter was used to collect the insects from the net before being transferred into pill vials with perforated lids. The vials were placed on ice to lower the temperature during transport. Third to fourth instar nymphs were also collected from Kurland using the active beating method. The nets were emptied into a tray (40 χ 50 cm) and the contents were evenly spread out before being turned upside down. The nymphs remained attached to the tray and were then transferred to transparent tubs with perforated lids for transport. Cyclopia longifolia seedlings which were due for transplanting were acquired from the Kurland nursery.
Experimental procedure
A completely randomised design with 21 treatments, including the control, replicated five times was used for the bioassay. The bioassay test was repeated twice on different dates with a fresh batch of fungal isolates and insects. The experimental setup comprised a modified assay chamber consisting of a sterilised 2-l soft drink transparent plastic bottle, which was cut at the bottom and used to cover the treated seedlings to prevent the insects from escaping and an inverted, transparent plastic container to help seal the bottom of the assay chamber. Seedlings of C. longifolia with a minimum number of 20 and a maximum number of 40 leaves were used for the bioassay. The seedlings were transplanted from trays into 250 ml disposable foam cups. The seedlings were first washed with distilled water, allowed to dry and then sprayed with 0.4 ml fungal suspension (107 conidia/ ml) using a spray bottle and left to dry at room temperature. The Control treatment was sprayed with distilled water.
Ten adult leafhoppers were introduced from the top which was sealed thereafter to prevent the insects from escaping. The lid of each chamber was briefly opened daily to prevent moisture from accumulating inside the chamber as this promotes the growth of saprophytic and opportunistic fungi (Oliver et al. 2011). Mortality was checked after seven days by counting the number of dead insects in each treatment. The bioassay was also conducted on the immature stages of the Molopopterus sp. using the same method as that described but with slight modifications. Transparent tubs (11 cm diameter χ 8 cm height) with perforated lids were used as bioassay chambers. Branches of C. longifolia were inserted into a wet floral foam in a Petri dish, then placed at the bottom of the tub. Ten crawling nymphs were then transferred to the bioassay chamber using a fine needle-nosed paint brush. Mortality was assessed as for the adults.
Data analysis
Abbott's (1925) formula was used to adjust for natural mortality. A one-way analysis of variance (ANOVA) was used to compare the differences in the mean mortality of Molopopterus sp. between 20 treatments of fungal isolates. A Bonferroni Least Significant Differences (LSD) test was used to separate the means. Data were analysed using R software version 4.0.0. One-way ANOVA was specified using the "stats" package. The Shapiro-Wilk and the Bartlett test were used to check for normality and homogeneity of variances, respectively. LSD tests were specified using the "agricolae" package (Mendiburu & Muhammad 2020).
RESULTS
Isolation and identification of entomopathogenic fungi
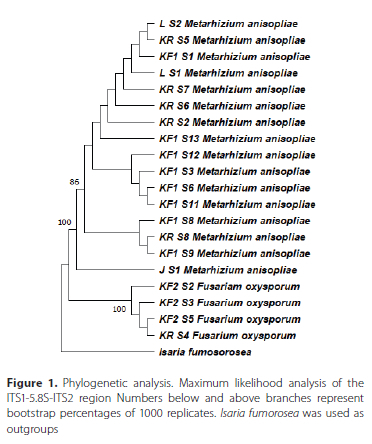
Twenty fungal isolates were recovered from 98 soil samples (20.04%). Fourteen isolates (70%) were recovered from 58 samples collected in planted fields while six isolates (30%) were recovered from 40 samples collected in the surrounding refugia. Metarhizium anisopliae was the most recovered species in both the field and refugia while Fusarium oxysporum Schlecht. emend. Snyder & Hansen (Hypocreales: Nectriaceae) was the other isolated fungal species. Most of the fungal isolates were isolated from Plettenberg Bay (17) while the remainder of the isolates originated from Sedgefield (2) and George (1) (Table 1). A maximum likelihood phylogenetic tree was drawn using only sequences that displayed some degree of variability (identical ITS1-5.8S-ITS2 regions with previously reported sequences excluded from the tree for clarity). Metarhizium and Fusarium isolates each formed their own clade (Figure 1).
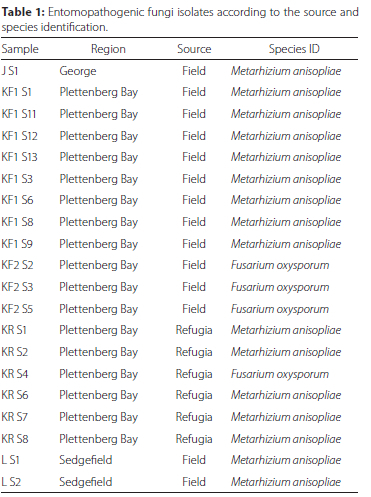
Fungal pathogenicity bioassays against Molopopterus sp.
All the isolates had over 85% conidia germination on SDA plates. Control treatments had low mortalities of 9 ± 1.7% and 6 ± 2.1% in adults and nymphs, respectively, and was used to control for natural mortality within each experimental treatment. The average adult mortality differed significantly between treatments (F19 179 = 16.892, p = 0.0021) (Figure 2). The highest mortality (72%) was reported for M. anisopliae KF1 S13, whilst the lowest mortality (11%) was reported for F. oxysporum KF2S2. Similarly, the average nymph mortality differed significantly between treatments (F19 179 = 5.463, p = 0.00097) (Figure 3). Metarhizium anisopliae isolates induced higher mortalities against both adults and nymphs (25-80%) compared to F. oxysporum isolates (10-60%). Metarhizium anisopliae isolates J S1, KF S3, KF S6, KF S11, KF S12 and KF S13 induced mortalities greater than 60% in both adults and nymphs.
DISCUSSION
A relatively low recovery rate of EPF from soil samples was consistent with findings by Goble et al. (2010) who recovered 21.53% from South African citrus orchards and Dlamini et al. (2020) who recovered 37% from deciduous fruits and grapevine orchards in the Western Cape Province of South Africa. However, higher recovery rates have been reported in other parts of the world. Bidochka et al. (1998) recovered 91% from temperate soils in Canada, and Keller et al. (2003) had a recovery rate of 96% from different habitats in Switzerland using the insect bait method in both natural and disturbed soils. The low recovery rate was also coupled with low species diversity as only two species of fungi were recovered. Dlamini et al. (2020) also recovered two species M. anisopliae and Beauveria bassiana. However, Abaajeh (2014), discovered six different fungal species in pomegranate farms in the Western Cape of which B. bassiana was the most common species.
This study rendered two fungal species important for use as biological control agents against this leafhopper (Molopopterus sp.) but did not detect any strains of the popularly reported B. bassiana. Occurrence and distribution of entomopathogenic fungi is influenced by several factors such as location, habitat type, soil properties and cropping system which may further influence recovery of isolates (Klingen et al. 2002; Asensio et al. 2003; Meyling & Eilenberg 2006). Differences in recovery could also be explained by different isolation protocols, variations in the bait insect species, the number of bait insects per sample, baiting temperature, and volume of soil. Galleria mellonella is the most commonly used insect species in the insect bait technique and is very sensitive to isolation of entomopathogenic fungi in the soil. Goble et al. (2010) recovered significantly more isolates from G. mellonella compared to Ceratitis capitata Wiedemann (Diptera Tephritidae) and Thaumatotibia leucotreta Meyrick (Lepidoptera: Tortricidae). The "insect bait" method was used in this study, however, mealworms, T. molitor, were used instead of the common G. mellonella. Albertyn et al. (2021), had a slightly higher recovery of 34.3% using T. molitor, therefore, bait insect species alone cannot explain low recovery rate. This then suggests that other factors are responsible for the low species diversity rather than the bait insect species used. Alternatively, sampled Honeybush plantations and their surrounding refugia may simply not contain other species of fungus.
The study showed that habitat type, cultivated land versus natural refugia, played a significant role in both the occurrence and recovery of fungal isolates. Soils harbouring Fusarium oxysporum were more commonly recovered from surrounding refugia, but the presence of M. anisopliae was more strongly associated with cultivated Honeybush fields. This is consistent with the findings of Bidochka et al. (1998) and Tkaczuk et al. (2013), who recovered more isolates and greater species richness in natural habitats over disturbed ecosystems. Occurrence of EPF is naturally associated with organic matter in the soil (Bidochka et al. 2001). This may be due to cation exchange in organic matter facilitating adsorption of fungal conidia (Inglis et al. 2012). However, this is in contrast to findings by Goble et al. (2010), who recovered more isolates from refugia than in citrus orchards. The difference could be explained by conventional pest management practices in citrus orchards which can influence occurrence of fungi in the soil. Honeybush tea, is a perennial crop that is organically grown; under certain conditions it may provide a microhabitat that supports a greater diversity of arthropod hosts in which fungi can multiply (Slabbert et al. 2019).
Recovery of F. oxysporum was quite unusual as many Fusarium spp. are well known for their role as plant pathogens and mycotoxin producers (O'Donnell et al. 2018). The genus Fusarium is well known to switch between different life stages and act on non-plant hosts (Van Diepeningen & De Hoog 2016; Santos et al. 2020). Research on Fusarium-insect interaction has been limited as they are generally characterised as opportunistic insect pathogens. However, some Fusarium spp. are reported to possess entomopathogenic properties (Santos et al. 2020). The Fusarium oxysporum species complex has been isolated from soil samples using the Galleria bait method (Sharma & Marques 2018; Santos et al. 2020). Abaajeh (2014), recovered seven Fusarium spp., in the Western Cape from citrus plantations, five of which were F. oxysporum isolates. Recently, Sharma and Marques (2018) did a review seeking to address Fusarium-insect pathogenicity under field conditions. Feng-Yan and Quing-Tao (1991), recovered 180 Fusarium isolates from 150 different insect cadavers including spiders. This demonstrates the potential of Fusarium spp. as potential biocontrol agents.
Different Fusarium spp. have shown low to high mortality when tested against lepidopterans (Ali-Shtayeh et al. 2003), while showing moderate to high mortality against Coleoptera and Hemiptera (Anwar et al. 2017; Sharma & Marques 2018). Ganassi et al. (2001) recorded 60% mortality induced by Fusarium spp. against the aphid, Schizaphis gramium Rondani (Hemiptera: Aphididae). Thangam et al. (2014) also recorded 45-60% mortality in mango leafhoppers following Fusarium spp. application. Moreover, Fusarium spp. have also been identified to produce Beauvericin, a cyclic depsipeptide and one of the major toxins produced by B. bassiana which exhibits insecticidal properties (Wang & Xu 2012; Wang et al. 2018). However, there is controversy as to whether they can adequately be classified as EPF. Navarro-Velasco et al. (2011), studied histological evidence to comprehend its infection process and concluded that they do possess insect pathogenicity properties. Fusarium oxysporum isolates induced low mortalities against Molopopterus sp., however, they may still retain potential as biocontrol agents against other Honeybush tea pests. Although Fusarium spp. have the potential for biocontrol, further evaluations concerning their safety and environmental impacts need to be undertaken before consideration as biocontrol agents.
Both adults and nymphs of Molopopterus sp. had similar responses to infection by the tested fungal pathogens with some isolates inducing mortalities over 60%. Discrepancies were also observed amongst isolates in the mortalities induced between adults and nymphs. According to Mochi et al. (2005), not all life stages are vulnerable to penetration by propagules; some are more vulnerable than others. Gul et al. (2015) conducted pathogenicity tests against different life stages of the peach fruit fly, Bactrocera zonata Saunders (Diptera: Tephritidae), and observed higher mortalities in adults than immature and pupal stages. Therefore, the life stage which is targeted can influence the probability of infection. Pupal stages are often most resistant, while nymphs and adults are most susceptible (Aatif et al. 2020). These discrepancies could be attributed to behavioural differences, coupled with inoculation technique used in this study (based on secondary pick-up). Moreover, the time of inoculation prior to ecdysis and the length of the intermoult period play a critical role. If moulting occurs shortly after inoculation, it may remove penetrating conidia before colonisation of the insect host (Boomsma et al. 2014).
Previous studies on pests in the order Hemiptera have recorded even higher mortalities using M. anisopliae. Sain et al. (2019), recorded high mortality (89%) on Bemisia tabaci Genadius (Hemiptera: Aleyrodidae) while, Bayissa et al. (2017), recorded comparable mortalities (73%) at 107 conidia/ml against aphid pest species of crucifers and okra which were consistent with the findings of this study. Tounou et al. (2003), discovered that increasing time of exposure in bioassays to 15 days resulted in even higher mortalities (97%) when they evaluated EPF against the green leafhopper Empoasca decipiens Paoli (Homoptera: Cicadellidae). Susceptibility of an insect is dependent on several factors that directly or indirectly impact pathogen performance.
In conclusion, the M. anisopliae isolates, J S1, KF S3, KF S11, KF S13, LS1 and LS2 are recommended for further evaluation as they caused the highest mortality in both adults and nymphs. However, further evaluation needs to occur before being considered for field application. This includes further characterisation of virulence (dose- and time-response) as well as semi-field trials to establish their performance under more realistic conditions, where several uncontrollable abiotic and biotic factors may influence their efficacy. Given that M. anisopliae may be capable of infecting other important pests of honeybush, determining whether this is possible is also warranted as it may promote the development of a biopesticide which can be targeted against more than just Molopopterus sp. in honeybush and other cultivated crops where this pest is problematic.
ACKNOWLEDGEMENTS
This work was supported by the South African Honeybush Tea Association through the Alternative Crop Fund of the Western Cape Government Department of Agriculture. Further funding for this work was provided by the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation of South Africa. Any opinion, finding, conclusion or recommendation expressed in this material is that of the authors and the NRF does not accept any liability in this regard.
ORCID IDS
Tapiwa G Mushore - https://orcid.org/0000-0002-6697-5236
Candice A Coombes - https://orcid.org/0000-0002-3868-0895
Martin P Hill - https://orcid.org/0000-0003-0579-5298
REFERENCES
Aatif HM, Hanif MS, Raheel M, Ferhan, M, Mansha MZ, Khan AA, Ullah, MI, Shakeel Q, ali S. 2020. Temperature dependent virulence of the entomopathogenic nematodes against immatures of the oriental fruit fly, Bactrocera dorsalis Hendel (Diptera: Tephritidae). Egyptian Journal of Biological Pest Control 30:42-48. https://doi.org/10.1186/s41938-020-00248-7 [ Links ]
Abaajeh AR. 2014. Evaluation of entomopathogenic fungi (ascomycota) for the control of Cydia pomonella (Lepidoptera: Tortricidae). Unpublished MSc thesis, Cape Penisula University of Technology.
Abbott WS. 1925. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology 18:265-267. https://doi.org/10.1093/jee/18.2.265a [ Links ]
Albertyn S, Moore SD, Marsberg T, Coombes CA, Hill MP. 2021. The influence of citrus orchard age on the ecology of entomopathogenic fungi and nematodes. Biocontrol Science and Technology 31: 80-96. https://doi.org/10.1080/09583157.2020.1830949 [ Links ]
Ali-Shtayeh MS, Mara'i, AB, Jamous RM. 2003. Distribution, occurrence and characterization of entomopathogenic fungi in agricultural soil in the Palestinian area. Mycopathologia 156:235-244. https://doi.org/10.1023/a:1023339103522 [ Links ]
Andow D. 1983. The extent of monoculture and its effects on insects. Agriculture, Ecosystems and Environment 9:25-35. https://doi.org/10.1016/0167-8809(83)90003-8 [ Links ]
Anwar W, Haider MS, Shadid AA, Mushtaq H, Hameed U, Reham MZ, Iqbal M J. 2017. Genetic diversity of Fusarium isolated from members of Sternorrhyncha (Hemiptera): Entomopathogens against Bemisia tabaci. Pakistan Journal of Zoology 49:639-645. https://doi.org/10.17582/journal.pjz/2017.49.2.639.645 [ Links ]
Asensio L, Carbonell T, Lopez-Jimenez JA, López-Llorca LV. 2003. Entomopathogenic fungi in soils from Alicante province [Spain]. Spanish Journal of Agricultural Research 1:37-45. https://doi.org/10.5424/sjar/2003013-33 [ Links ]
Barra P, Rosso L, Nesci A, Etcheverry M. 2012. Isolation and identification of entomopathogenic fungi and their evaluation against Tribolium confusum, Sitophilus zeamais, and Rhyzopertha dominica in stored maize. Journal of Pest Science 86:217-226. https://doi.org/10.1007/s10340-012-0460-z. [ Links ]
Bayissa W, Ekesi S, Mohamed SA, Kaaya GP, Wagacha JM, Hanna R, Maniania NK. 2017. Selection of fungal isolates for virulence against three aphid pest species of crucifers and okra. Journal of Pest Science 90:355-368. https://doi.org/10.1007/s10340-016-0781-4. [ Links ]
Bidochka MJ, Kamp AM, Lavender M, Dekoning J, Croos ADE. 2001. Habitat association in two genetic groups of the insect-pathogenic fungus. Society 67:1335-1342. https://doi.org/10.1128/AEM.67.3.1335-1342.2001 [ Links ]
Bidochka NJ, Kasperski JE, Wild GA. 1998. Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from temperate and near-northern habitats. Canada Journal of Botany 76:1198-1204. https://doi.org/10.1139/b98-115 [ Links ]
Boomsma JJ, Jensen AB, Meyling NV, Eilenberg J. 2014. Evolutionary interaction networks of insect pathogenic Fungi. Annual Review of Entomology 59:171-220. https://doi.org/10.1146/annurev-ento-011613-162054 [ Links ]
Department of Agriculture, Forestry and Fisheries (DAFF). 2014. Honebush Tea. Pretoria. [accessed 20/01/2022]. https://www.dalrrd.gov.za/Portals/0/Brochures%20and%20Production%20guidelines/honey%20bush%20tea.pdf
Dlamini BE, Malan AP, Addison P. 2020. Entomopathogens from agricultural soil and their potential to control the banded fruit weevil, Phlyctinus callosus (Schönherr) (Coleoptera: Curculionidae). African Entomology 28:374-384. https://doi.org/10.4001/003.028.0374 [ Links ]
Feng-Yan B, Quing-Tao C. 1991. Fusarium species on some insects from China. Acta Mycologica 10:120-128. https://doi.org/10.3390/pathogens7040093 [ Links ]
Ganassi S, Moretti A, Stornelli C, Fratello B, Bonvicini Pagliai AM, Logrieco A, Sabatini MA. 2001. Effect of Fusarium, Paecilomyces and Trichoderma formulations against aphid Schizaphis graminum. Mycopathologia 151:131-138. https://doi.org/10.1023/A:1017940604692 [ Links ]
Goble TA, Dames JF, Hill MP, Moore SD. 2010. The effects of farming system, habitat type and bait type on the isolation of entomopathogenic fungi from citrus soils in the Eastern Cape Province, South Africa. BioControl 55:399-412. https://doi.org/10.1007/s10526-009-9259-0 [ Links ]
Gul HT, Freed S, Akmal M, Malik MN. 2015. Vulnerability of different life stages of Bactrocera zonata (Tephritidae: Diptera) against entomogenous fungi. Pakistan Journal of Zoology 47:307-317. [ Links ]
Habibi J, Backus EA, Coudron TA, Brandt SL. 2001. Effect of different host substrates on hemipteran salivary protein profiles. Entomologia Experimentalis et Applicata 98:369-375. https://doi.org/10.1023/A:1018971827110 [ Links ]
Hatting JL. 2017. Major insect pests and their natural enemies associated with cultivation of Rooibos, Aspalathus linearis ( Burm . f .) R. Dahlgren , in South Africa : A review. South African Journal of Botany 110:118-123. https://doi.org/10.1016/j.sajb.2016.07.01 [ Links ]
Hazarika L, Puzari KC. 2001. Microbials in tea pest management. Ignacimuthu I, Sen A, editors. Microbials in Insect Pest Management. Oxford, New Dehli. 98-104 pp.
Hussain A, Rizwan-Ul-Haq M, Al-Ayedh H, Al-Jabr A. 2014. Mycoinsecticides: Potential and future perspective. Recent Patents on Food, Nutrition & Agriculture 6:45-53. https://doi.org/10.2174/2212798406666140613113905 [ Links ]
Inglis GD, Enkerli J, Goettel MS. 2012. Laboratory techniques used for entomopathogenic fungi. Hypocreales. Manual of Techniques in Invertebrate Pathology (2nd ed). Elsevier. 189-253 pp.
Joubert E, Joubert ME, Bester C, De Beer D, De Lange JH. 2011. Honeybush (Cyclopia spp.): From local cottage industry to global markets - The catalytic and supporting role of research. South African Journal of Botany 77:887-907. https://doi.org/10.1016/j.sajb.2011.05.014 [ Links ]
Keller S, Kessler P, Schweizer C. 2003. Distribution of insect pathogenic soil fungi in Switzerland with special reference to Beauveria brongniartii and Metharhizium anisopliae. BioControl 48:307-319. https://doi.org/10.1023/A:1023646207455. [ Links ]
Khan S, Guo L, Maimaiti Y, Mijit M, Qiu D. 2012. Entomopathogenic fungi as microbial biocontrol agent. Molecular Plant Breeding 3:6379. https://doi.org/10.5376/mpb.2012.03.0007 [ Links ]
Klingen I, Eilenberg J, Meadow R. 2002. Effects of farming system, field margins and bait insect on the occurrence of insect pathogenic fungi in soils. Agriculture, Ecosystems and Environment 91:191-198. https://doi.org/10.1016/s0167-8809(01)00227-4 [ Links ]
Marzachi C, Veratti F, Bosco D. 1998. Direct PCR detection of phytoplasmas in experimentally infected insects. Annals of Applied Biology 133:45-54. https://doi.org/10.1111/j.1744-7348.1998.tb05801.x [ Links ]
Mascarin GM, Lopes RB, Delalibera Í, Fernandes ÉKK, Luz C, Faria M. 2019. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. Journal of Invertebrate Pathology 165:46-53. https://doi.org/10.1016/j.jip.2018.01.001 [ Links ]
Mbangcolo MM. 2008. Aspects of honeybush tea (Cyclopia Species) propagation. [MSc thesis]. Stellenbosch: Stellenbosch University [ Links ]
Mcgregor GK. 2018. The wild Honeybush harvesting field guide. Capetown. [accessed 7 May 2019]. https://gouritz.com/wp-content/uploads/2019/02/Honeybush-harvesting-Eng_Wild-honey bush-harvesting-field-guide_McGregor-2018-compressed.pdf
Mendiburu F, Muhammad Y. 2020. agricolae: Statistical procedures for agricultural research.R package version 1.4.0.
Metcalf E, Sephton M, Lands L, Brunette D. 2018. Overview of the keurboom moth' (Leto venus) and its Impact on Cultivated Cyclopia ( Honeybush ) plantations in South Africa. Grounded, Cape Town. [accessed 21 May 2019]. https://grounded.co.za/what-is-so-great-about-honeybush/
Meyling N. 2007. Methods for isolating entomopathogenic fungi from the soil environment. Department of Ecology, Faculty of Life Sciences, University of Copenhagen, Denmark. [accessed: 12 January 2021] http://orgprints.org/11200/1/11200.pdf
Meyling NV, Elenberg J. 2006. Occurrence and distribution of soil borne entomopathogenic fungi within a single organic agroecosystem. Agriculture, Ecosystems and Environment 113:336-341. https://doi.org/10.1016/j.agee.2005.10.011 [ Links ]
Mochi DA, Monteiro CA, Barbosa JC. 2005. Action of pesticides to Metarhizium anisopliae in soil. Neotropical Entomology 34:961971. https://doi.org/10.1590/S1519-566X2005000600013 [ Links ]
Navarro-Velasco GY, Prados-Rosales RC, Oritz-Urquiza A, Quesada-Moraga E, Di Pietro A. 2011. Gallleria mallonella as model host for the trans-kingndom pathogen Fusarium oxysprorum. Fungal Genetics and Biology 48:1124-1129. https://doi.org/10.1016/j.fgb.2011.08.004 [ Links ]
North MS, Joubert E, Beer DE, Kock K, Joubert ME. 2017. Effect of harvest date on growth, production and quality of Honeybush (Cyclopia genistoides and C. subternata). South African Journal of Botany 110:132-137. https://doi.org/10.1016/j.sajb.2016.08.002 [ Links ]
O'Donnell K, Mccormick SP, Busman M, Proctor RH, Todd JW, Gail D, David MG, Johanna FA, John PR. 2018. Marasas et al. 1984 "Toxigenic Fusarium Species: Identity and Mycotoxicology" revisited. Mycologia 52:1-23. https://doi.org/10.1080/00275514.2018.1519773 [ Links ]
Olivier CY, Vincent C, Saguez J, Galka B, Weintraub PG, Maixner M. (2012). Leafhoppers and Planthoppers: Their Bionomics, Pathogen Transmission and Management in Vineyards, In Bostanian NJ, Vincent C, Isaacs R, editors Arthropod Management in Vineyards: Pests, Approaches, and Future Directions, Springer Dordrecht, The Netherlands, p. 253-270.
Quesada-Moraga E, Navas-Cortés JA, Maranhao EAA, Ortiz-Urquiza A, Santiago-Álvarez C. 2007. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycological Research 111:947-966. https://doi.org/10.1016/j.mycres.2007.06.006 [ Links ]
Rath AC, Koen TB, Yip HY. 1992. The influence of abiotic factors on the distribution and abundance of Metarhizium anisopliae in Tasmanian pasture soils. Mycological Research 96:378-384. https://doi.org/10.1016/S0953-7562(09)80956-8 [ Links ]
Sain SK, Monga D, Kumar R, Nagrale DT, Kranthi S, Kranthi KR. 2019. Comparative effectiveness of bioassay methods in identifying the most virulent entomopathogenic fungal strains to control Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Egyptian Journal of Biological Pest Control 29:1-11. https://doi.org/10.1186/s41938-019-0130-z [ Links ]
Santos AC, Da S, Diniz AG, Tiago PV, Oliveira NT . 2020. Entomopathogenic Fusarium species: a review of their potential for the biological control of insects, implications and prospects. Fungal Biology Reviews 34:41-57. https://doi.org/10.1016/jfbr.2019.12.002 [ Links ]
Schutte AL. 1995. Systematics of the genus Cyclopia vent. (Fabaceae, Podalyrieae). Edinburgh Journal of Botany 54:125-170. https://doi.org/10.1017/S0960428600004005 [ Links ]
Sharma L, Marques G. 2018. Fusarium, an entomopathogen-a myth or reality? Pathogens 7: 93-100. https://doi.org/10.3390/pathogens7040093. [ Links ]
Slabbert EL, Malgas RR, Veldtman R, Addison P. 2019. Honeybush (Cyclopia spp.) phenology and associated arthropod diversity in the Overberg region, South Africa. Bothalia 49:a2430. https://doi.org/10.4102/abc.v49i1.2430 [ Links ]
Thangam SD, Selvakumar G, Verghese A, Kamala Jayanthi PD. 2014. Natural mycosis of mango leafhoppers (Cicadellidae: Hemiptera) by Fusarium sp. Biocontrol Science and Technology 24:229-232. https://doi.org/10.1080/09583157.2013.851171 [ Links ]
Thomas MB, Read AF. 2007. Can fungal biopesticides control malaria? Nature Reviews Microbiology 5:377-383. https://doi.org/10.1038/nrmicro1638 [ Links ]
Tkaczuk C, Krzyczkowski T, Wegensteiner R. 2013. The occurrence of entomopathogenic fungi in soils from mid-field woodlots and adjacent small-scale arable fields. Acta Mycologica 47:191-202. https://doi.org/10.5586/am.2012.024 [ Links ]
Tounou AK, Agboka K, Poehling HM, Raupach K, Langewald J, Zimmermann G, Borgemeister C. 2003. Evaluation of the entomopathogenic fungi Metarhizium anisopliae and Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) for control of the Green Leafhopper Empoasca decipiens (Homoptera: Cicadellidae) and potential side effects on the egg parasitoid Anagrus atomus (Hymenoptera: Mymaridae). Biocontrol Science and Technology 13:715-728. https://doi.org/10.1080/09583150310001606534 [ Links ]
van Diepeningen AD, de Hoog GS. (2016). Challenges in Fusarium, a Trans-Kingdom Pathogen. Mycopathologia, 181:161-163. https://doi.org/10.1007/s11046-016-9993-7 [ Links ]
Wang Q, Gong X, Li P, Lai D, Zhou L. 2018. Structural diversity and biological activities of cyclic depsipeptides form fungi. Molecules 23:169-172. https://doi.org/10.3390/molecules23010169 [ Links ]
Wang Q, Xu L. 2012. Beauvericin, a bioctive compound produced by fungi: A short review. Molecules 17:2367-2377. https://doi.org/10.3390/molecules17032367. [ Links ]
Zimmermann G. 2007. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Science and Technology 17:879-920. https://doi.org/10.1080/09583150701593963 [ Links ]
 Correspondence:
Correspondence:
TG Mushore
Email: mushoretapiwa@gmail.com
Received: 22 June 2022
Accepted: 10 February 2023














